Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies
- PMID: 15026791
- PMCID: PMC2409647
- DOI: 10.1038/sj.bjc.6601663
Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies
Abstract
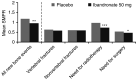
Although intravenous (i.v.) bisphosphonates are the standard of care for metastatic bone disease, they are less than ideal for many patients due to infusion-related adverse events (AEs), an increased risk of renal toxicity and the inconvenience of regular hospital visits. The use of oral bisphosphonate therapy is limited by concerns over efficacy and gastrointestinal (GI) side effects. There remains a clinical need for an oral bisphosphonate that offers equivalent efficacy to i.v. bisphosphonates, good tolerability and dosing convenience. Oral ibandronate, a highly potent, third-generation aminobisphosphonate, has been evaluated in phase III clinical trials of patients with bone metastases from breast cancer. In two pooled phase III studies, patients with breast cancer and bone metastases were randomised to receive oral ibandronate 50 mg (n=287) or placebo (n=277) once daily for up to 96 weeks. The primary end point was the skeletal morbidity period rate (SMPR), defined as the number of 12-week periods with new skeletal complications. Multivariate Poisson's regression analysis was used to assess the relative risk of skeletal-related events in each treatment group during the study period. Oral ibandronate 50 mg significantly reduced the mean SMPR compared with placebo (0.95 vs 1.18, P=0.004). There was a significant reduction in the mean number of events requiring radiotherapy (0.73 vs 0.98, P<0.001) and events requiring surgery (0.47 vs 0.53, P=0.037). Poisson's regression analysis confirmed that oral ibandronate significantly reduced the risk of a skeletal event compared with placebo (hazard ratio 0.62, 95% CI=0.48, 0.79; P=0.0001). The incidence of mild treatment-related upper GI AEs was slightly higher in the oral ibandronate 50 mg group compared with placebo, but very few serious drug-related AEs were reported. Oral ibandronate 50 mg is an effective, well-tolerated and convenient treatment for the prevention of skeletal complications of metastatic bone disease.
Figures
References
-
- Body JJ (2003) Effectiveness and cost of bisphosphonate therapy in tumor bone disease. Cancer 97(Suppl): 859–865 - PubMed
-
- Body JJ, Diel IJ, Lichinitser MR, Kreuser ED, Dornoff W, Gorbunova VA, Budde M, Bergström B (2003a) On behalf of the MF 4265 Study Group. Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol 14: 1399–1405 - PubMed
-
- Body JJ, Diel I, Tripathy D, Bergstrom B (2003b) Impact of ibandronate on bone pain in patients with metastatic bone disease from breast cancer. Support Care Cancer 11: 395–396 (abstract A-34)
-
- Body JJ, Kanis J, Diel I, Bergstrom B (2003c) Risk reductions in metastatic breast cancer: multivariate Poisson regression analyses of oral and i.v. ibandronate. Proc ASCO 22: 46 (abstract 184)
-
- Diel I, Bell R, Tripathy D, Body JJ, Bergström B (2003) Renal safety of oral and intravenous ibandronate in metastatic bone disease: phase III clinical trial results. Support Care Cancer 11: 415 (abstract A-106)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical