Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, randomised, phase III trials
- PMID: 15026800
- PMCID: PMC2409640
- DOI: 10.1038/sj.bjc.6601676
Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, randomised, phase III trials
Abstract
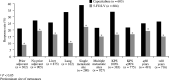
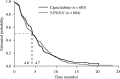
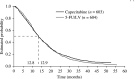
This study evaluates the efficacy of capecitabine using data from a large, well-characterised population of patients with metastatic colorectal cancer (mCRC) treated in two identically designed phase III studies. A total of 1207 patients with previously untreated mCRC were randomised to either oral capecitabine (1250 mg m(-2) twice daily, days 1-14 every 21 days; n=603) or intravenous (i.v.) bolus 5-fluorouracil/leucovorin (5-FU/LV; Mayo Clinic regimen; n=604). Capecitabine demonstrated a statistically significant superior response rate compared with 5-FU/LV (26 vs 17%; P<0.0002). Subgroup analysis demonstrated that capecitabine consistently resulted in superior response rates (P<0.05), even in patient subgroups with poor prognostic indicators. The median time to response and duration of response were similar and time to progression (TTP) was equivalent in the two arms (hazard ratio (HR) 0.997, 95% confidence interval (CI) 0.885-1.123, P=0.95; median 4.6 vs 4.7 months with capecitabine and 5-FU/LV, respectively). Multivariate Cox regression analysis identified younger age, liver metastases, multiple metastases and poor Karnofsky Performance Status as independent prognostic indicators for poor TTP. Overall survival was equivalent in the two arms (HR 0.95, 95% CI 0.84-1.06, P=0.48; median 12.9 vs 12.8 months, respectively). Capecitabine results in superior response rate, equivalent TTP and overall survival, an improved safety profile and improved convenience compared with i.v. 5-FU/LV as first-line treatment for MCRC. For patients in whom fluoropyrimidine monotherapy is indicated, capecitabine should be strongly considered. Following encouraging results from phase I and II trials, randomised trials are evaluating capecitabine in combination with irinotecan, oxaliplatin and radiotherapy. Capecitabine is a suitable replacement for i.v. 5-FU as the backbone of colorectal cancer therapy.
Figures
References
-
- André T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, Beerblock K, Bouche O, Carola E, Merrouche Y, Morvan F, Dupont-Andre G, de Gramont A (1999) Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol 17: 3560–3568 - PubMed
-
- Borner M, Dietrich D, Popescu R, Wernli M, Roth AD, Saletti P, Rauch DP, Herrmann R, Koberle D, Pestalozzi B, Honegger H, Castiglione-Gertsch M, Goldhirsch A (2003) A randomized phase II trial of capecitabine (CAP) and two different schedules of irinotecan (IRI) in first-line treatment of metastatic colorectal cancer (MCC). Proc Am Soc Clin Oncol 22: 266 (Abstract 1068)
-
- Borner M, Schöffski P, de Wit R, Caponigro F, Comella G, Sulkes A, Greim G, Peters GJ, van der Born K, Wanders J, de Boer RF, Martin C, Fumoleau P (2002) Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer 38: 349–358 - PubMed
-
- Cassidy J, Twelves C, Van Cutsem E, Hoff P, Bajetta E, Boyer M, Bugat R, Burger U, Garin A, Graeven U, McKendric J, Maroun J, Marshall J, Osterwalder B, Perez-Manga G, Rosso R, Rougier P, Schilsky RL, Capecitabine Colorectal Cancer Study Group (2002) First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with i.v. 5-fluorouracil (5-FU)/leucovorin. Ann Oncol 13: 566–575 - PubMed
-
- Cunningham D, Pyrhönen S, James RD, Punt CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham CA, Awad L, Jacques C, Herait P (1998) Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352: 1413–1418 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical