Toll-like receptor 2 (TLR2) and TLR4 are present inside human dendritic cells, associated with microtubules and the Golgi apparatus but are not detectable on the cell surface: integrity of microtubules is required for interleukin-12 production in response to internalized bacteria
- PMID: 15027902
- PMCID: PMC1782406
- DOI: 10.1111/j.0019-2805.2003.01803.x
Toll-like receptor 2 (TLR2) and TLR4 are present inside human dendritic cells, associated with microtubules and the Golgi apparatus but are not detectable on the cell surface: integrity of microtubules is required for interleukin-12 production in response to internalized bacteria
Abstract
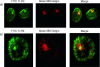
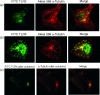
The activation of dendritic cells (DCs) by microbes is mediated by pattern recognition receptors including the Toll-like receptors (TLR). Bacterial lipopolysaccharide acts via TLR4 whereas peptidoglycan and lipoprotein responses are mediated by TLR2. It is generally accepted that TLR binding to microbes occurs at the cell surface but this has not been directly demonstrated for human DCs. We show here that TLR2 and TLR4 are expressed inside DCs in an abundant tubulovesicular pattern with a focus of intense staining adjacent to the nucleus. In contrast, there was no detectable expression on the cell surface. TLR2 and TLR4 were readily found both intracellularly and on the surface of monocytes. They were shown to be closely associated with the Golgi complex and colocalized with alpha-tubulin, displaying a high focal concentration at the microtubule organizing centre. Alignment of TLR2 and TLR4 with microtubules was observed, suggesting that microtubules serve as transport tracks for TLR vesicles. Depolymerization of the microtubule network disrupted the intracellular expression of TLR2 and TLR4 and profoundly inhibited interleukin-12 (IL-12) production in response to Neisseria meningitidis but did not prevent phagocytosis. These data are consistent with the bacterial signalling through TLR2 and TLR4 required for IL-12 production occurring inside DCs after phagocytosis.
Figures


References
-
- Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999;19:12–25. - PubMed
-
- Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. - PubMed
-
- Muzio M, Mantovani A. The Toll receptor family. Allergy. 2001;56:103–8. - PubMed
-
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. - PubMed
-
- Beutler B. Toll-like receptors: how they work and what they do. Curr Opin Hematol. 2002;9:2–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
