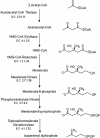
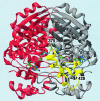
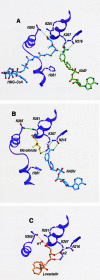
Class II 3-hydroxy-3-methylglutaryl coenzyme A reductases
- PMID: 15028676
- PMCID: PMC374403
- DOI: 10.1128/JB.186.7.1927-1932.2004
Class II 3-hydroxy-3-methylglutaryl coenzyme A reductases
Figures
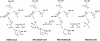

References
-
- Bochar, D. A., J. A. Friesen, C. V. Stauffacher, and V. W. Rodwell. 1999. Biosynthesis of mevalonic acid from acetyl-CoA, p. 15-44. In D. Cane (ed.), Comprehensive natural products chemistry, vol. 2. Isoprenoids, including carotenoids and steroids. Pergamon Press, Oxford, United Kingdom.
-
- Bochar, D. A., C. V. Stauffacher, and V. W. Rodwell. 1999. Sequence comparisons reveal two classes of 3-hydroxy-3-methyl coenzyme A reductase. Mol. Genet. Metab. 66:122-127. - PubMed
-
- Bochar, D. A., C. V. Stauffacher, and V. W. Rodwell. 1999. Investigation of the conserved lysines of Syrian hamster 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochemistry 38:15848-15852. - PubMed
-
- Bochar, D. A., L. Tabernero, C. V. Stauffacher, and V. W. Rodwell. 1999. Aminoethylcysteine can replace the active site lysine of Pseudomonas mevalonii 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochemistry 38:8879-8883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

