Sortase B, a new class of sortase in Listeria monocytogenes
- PMID: 15028680
- PMCID: PMC374393
- DOI: 10.1128/JB.186.7.1972-1982.2004
Sortase B, a new class of sortase in Listeria monocytogenes
Abstract
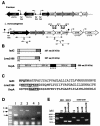
Sortases are transamidases that covalently link proteins to the peptidoglycan of gram-positive bacteria. The genome of the pathogenic bacterium Listeria monocytogenes encodes two sortases genes, srtA and srtB. The srtA gene product anchors internalin and some other LPXTG-containing proteins to the listerial surface. Here, we focus on the role of the second sortase, SrtB. Whereas SrtA acts on most of the proteins in the peptidoglycan fraction, SrtB appears to target minor amounts of surface polypeptides. We identified one of the SrtB-anchored proteins as the virulence factor SvpA, a surface-exposed protein which does not contain the LPXTG motif. Therefore, as in Staphylococcus aureus, the listerial SrtB represents a second class of sortase in L. monocytogenes, involved in the attachment of a subset of proteins to the cell wall, most likely by recognizing an NXZTN sorting motif. The DeltasrtB mutant strain does not have defects in bacterial entry, growth, or motility in tissue-cultured cells and does not show attenuated virulence in mice. SrtB-mediated anchoring could therefore be required to anchor surface proteins involved in the adaptation of this microorganism to different environmental conditions.
Figures





References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. A. Smith, J. G. Seidman, and K. Struhl. 1990. Current protocols in molecular biology. Wiley Interscience, New York, N.Y.
-
- Berche, P. 1995. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb. Pathog. 18:323-336. - PubMed
-
- Bierne, H., S. K. Mazmanian, M. Trost, M. G. Pucciarelli, G. Liu, P. Dehoux, L. Jansch, F. G. Portillo, O. Schneewind, and P. Cossart. 2002. Inactivation of the srtA gene in Listeria monocytogenes inhibits anchoring of surface proteins and affects virulence. Mol. Microbiol. 43:869-881. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

