Interlimb transfer of novel inertial dynamics is asymmetrical
- PMID: 15028745
- PMCID: PMC10709821
- DOI: 10.1152/jn.00960.2003
Interlimb transfer of novel inertial dynamics is asymmetrical
Abstract
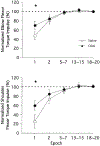
Mechanisms underlying interlimb transfer of adaptation to visuomotor rotations have recently been explored in depth. However, little data are available regarding interlimb transfer of adaptation to novel inertial dynamics. The present study thus investigated interlimb transfer of dynamics by examining the effect of initial training with one arm on subsequent performance with the other in adaptation to a 1.5-kg mass attached eccentrically to the forearm. Using inverse dynamic analysis, we examined the changes in torque strategies associated with adaptation to the extra mass, and with interlimb transfer of that adaptation. Following initial training with the dominant arm, nondominant arm performance improved substantially in terms of linearity and initial direction control as compared with naïve performance. However, initial training with the nondominant arm had no effect on subsequent performance with the dominant arm. Inverse dynamic analysis revealed that improvements in kinematics were implemented by increasing flexor muscle torques at the elbow to counter load-induced increases in extensor interaction torques as well as increasing flexor muscle torques at the shoulder to counter the extensor actions of elbow muscle torque. Following opposite arm adaptation, the nondominant arm adopted this dynamic strategy early in adaptation. These findings suggest that dominant arm adaptation to novel inertial dynamics leads to information that can be accessed and utilized by the opposite arm controller, but not vice versa. When compared with our previous findings on interlimb transfer of visuomotor rotations, our current findings suggest that adaptations to visuomotor and dynamic transformations are mediated by distinct neural mechanisms.
Figures






References
-
- Clower DM, Hoffman JM, Votaw JR, Faber TL, Woods RP , and Alexander GE. Role of posterior parietal cortex in the recalibration of visually guided reaching. Nature 383: 618–621, 1996. - PubMed
-
- Cohen MM. Visual feedback, distribution of practice, and intermanual transfer of prism aftereffects. Percept Psychophys 14: 401–406, 1973. - PubMed
-
- Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS , and Shadmehr R. Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89: 168–176, 2003. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

