Aberrant cellular behavior of mutant torsinA implicates nuclear envelope dysfunction in DYT1 dystonia
- PMID: 15028751
- PMCID: PMC6729521
- DOI: 10.1523/JNEUROSCI.4461-03.2004
Aberrant cellular behavior of mutant torsinA implicates nuclear envelope dysfunction in DYT1 dystonia
Abstract
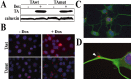
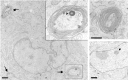
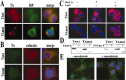
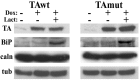
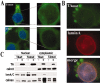
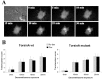
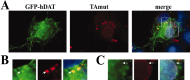
Torsion dystonia-1 (DYT1) dystonia, the most common inherited form of dystonia, is caused by a three base pair deletion that eliminates a single amino acid from the disease protein, torsinA. TorsinA is an "AAA" protein thought to reside in the endoplasmic reticulum (ER), yet both its cellular function and the basis for neuronal dysfunction in DYT1 remain unknown. A clue to disease pathogenesis is the fact that mutant, but not wild-type, torsinA forms membranous inclusions in cell culture. To explore the pathobiology of DYT1 dystonia, we generated PC12 neural cell lines that inducibly express wild-type or mutant torsinA. Although in this model torsinA displays some properties consistent with ER localization, mutant torsinA also accumulates in the nuclear envelope (NE), a structure contiguous with cytoplasmic ER. Consistent with this, membranous inclusions formed by mutant torsinA are shown to derive not from the ER, as thought previously, but from the NE. We demonstrate further that torsinA forms different disulfide-linked complexes that may be linked functionally to subcellular localization in the NE versus cytoplasmic ER. Despite mutant TA accumulation in NE structures, nucleocytoplasmic transport of a reporter protein was unaffected. These findings, together with parallel studies failing to demonstrate perturbation of ER function, implicate the NE as a primary site of dysfunction in DYT1. DYT1 dystonia can be added to the growing list of inherited neurological disorders involving the NE.
Figures


References
-
- Augood SJ, Hollingsworth Z, Albers DS, Yang L, Leung JC, Muller B, Klein C, Breakefield XO, Standaert DG (2002) Dopamine transmission in DYT1 dystonia: a biochemical and autoradiographical study. Neurology 59: 445–448. - PubMed
-
- Basham SE, Rose LS (2001) The Caenorhabditis elegans polarity gene ooc-5 encodes a Torsin-related protein of the AAA ATPase superfamily. Development 128: 4645–4656. - PubMed
-
- Berciano MT, Fernandez R, Pena E, Calle E, Villagra NT, Rodriguez-Rey JC, Lafarga M (2000) Formation of intranuclear crystalloids and proliferation of the smooth endoplasmic reticulum in Schwann cells induced by tellurium treatment: association with overexpression of HMG CoA reductase and HMG CoA synthase mRNA. Glia 29: 246–259. - PubMed
-
- Breakefield XO, Kamm C, Hanson PI (2001) TorsinA: movement at many levels. Neuron 31: 9–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
