Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways
- PMID: 15028759
- PMCID: PMC6729526
- DOI: 10.1523/JNEUROSCI.5377-03.2004
Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways
Abstract
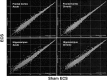
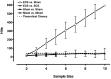
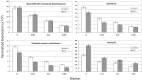
Electroconvulsive therapy (ECT) remains the treatment of choice for drug-resistant patients with depressive disorders, yet the mechanism for its efficacy remains unknown. Gene transcription changes were measured in the frontal cortex and hippocampus of rats subjected to sham seizures or to 1 or 10 electroconvulsive seizures (ECS), a model of ECT. Among the 3500-4400 RNA sequences detected in each sample, ECS increased by 1.5- to 11-fold or decreased by at least 34% the expression of 120 unique genes. The hippocampus produced more than three times the number of gene changes seen in the cortex, and many hippocampal gene changes persisted with chronic ECS, unlike in the cortex. Among the 120 genes, 77 have not been reported in previous studies of ECS or seizure responses, and 39 were confirmed among 59 studied by quantitative real time PCR. Another 19 genes, 10 previously unreported, changed by <1.5-fold but with very high significance. Multiple genes were identified within distinct pathways, including the BDNF-MAP kinase-cAMP-cAMP response element-binding protein pathway (15 genes), the arachidonic acid pathway (5 genes), and more than 10 genes in each of the immediate-early gene, neurogenesis, and exercise response gene groups. Neurogenesis, neurite outgrowth, and neuronal plasticity associated with BDNF, glutamate, and cAMP-protein kinase A signaling pathways may mediate the antidepressant effects of ECT in humans. These genes, and others that increase only with chronic ECS such as neuropeptide Y and thyrotropin-releasing hormone, may provide novel ways to select drugs for the treatment of depression and mimic the rapid effectiveness of ECT.
Figures

References
-
- Altar CA (1999) Neurotrophins and depression. Trends Pharmacol Sci 20: 59–61. - PubMed
-
- Altar CA, Whitehead RE, Chen R, Wortwein G, Madsen TR (2003) Effects of electroconvulsive seizure and antidepressants on BDNF protein content in brain. Biol Psych 54: 703–709. - PubMed
-
- Austin MC, Whitehead RE, Edgar CL, Janosky JE, Lewis DA (2002) Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience 114: 807–815. - PubMed
-
- Baik EJ, Kim EJ, Lee SH, Moon C (1999) Cyclooxygenase-2 selective inhibitors aggravate kainic acid induced seizure and neuronal cell death in the hippocampus. Brain Res 843: 118–129. - PubMed
-
- Barnea A, Roberts J (2001) Induction of functional and morphological expression of NPY in cortical cultures by BDNF: evidence for a requirement of ERK-dependent and ERK-independent mechanisms. Brain Res 919: 57–69. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
