A nonsense mutation of the sodium channel gene SCN2A in a patient with intractable epilepsy and mental decline
- PMID: 15028761
- PMCID: PMC6729532
- DOI: 10.1523/JNEUROSCI.3089-03.2004
A nonsense mutation of the sodium channel gene SCN2A in a patient with intractable epilepsy and mental decline
Abstract
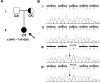
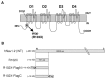
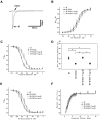
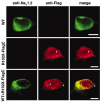
Mutations, exclusively missense, of voltage-gated sodium channel alpha subunit type 1 (SCN1A) and type 2 (SCN2A) genes were reported in patients with idiopathic epilepsy: generalized epilepsy with febrile seizures plus. Nonsense and frameshift mutations of SCN1A, by contrast, were identified in intractable epilepsy: severe myoclonic epilepsy in infancy (SMEI). Here we describe a first nonsense mutation of SCN2A in a patient with intractable epilepsy and severe mental decline. The phenotype is similar to SMEI but distinct because of partial epilepsy, delayed onset (1 year 7 months), and absence of temperature sensitivity. A mutational analysis revealed that the patient had a heterozygous de novo nonsense mutation R102X of SCN2A. Patch-clamp analysis of Na(v)1.2 wild-type channels and the R102X mutant protein coexpressed in human embryonic kidney 293 cells showed that the truncated mutant protein shifted the voltage dependence of inactivation of wild-type channels in the hyperpolarizing direction. Analysis of the subcellular localization of R102X truncated protein suggested that its dominant negative effect could arise from direct or indirect cytoskeletal interactions of the mutant protein. Haploinsufficiency of Na(v)1.2 protein is one plausible explanation for the pathology of this patient; however, our biophysical findings suggest that the R102X truncated protein exerts a dominant negative effect leading to the patient's intractable epilepsy.
Figures



References
-
- Ahmed CM, Ware DH, Lee SC, Patten CD, Ferrer-Montiel AV, Schinder AF, McPherson JD, Wagner-McPherson CB, Wasmuth JJ, Evans GA, Montal M (1992) Primary structure, chromosomal localization, and functional expression of a voltage-gated sodium channel from human brain. Proc Natl Acad Sci USA 89: 8220–8224. - PMC - PubMed
-
- Akai J, Makita N, Sakurada H, Shirai N, Ueda K, Kitabatake A, Nakazawa K, Kimura A, Hiraoka M (2000) A novel SCN5A mutation associated with idiopathic ventricular fibrillation without typical ECG findings of Brugada syndrome. FEBS Lett 479: 29–34. - PubMed
-
- Alekov AK, Rahman MM, Mitrovic N, Lehmann-Horn F, Lerche H (2001) Enhanced inactivation and acceleration of activation of the sodium channel associated with epilepsy in man. Eur J Neurosci 13: 2171–2176. - PubMed
-
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud'homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E (2001) First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet 28: 46–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
