Serologic and molecular biologic methods for SARS-associated coronavirus infection, Taiwan
- PMID: 15030702
- PMCID: PMC3322922
- DOI: 10.3201/eid1002.030731
Serologic and molecular biologic methods for SARS-associated coronavirus infection, Taiwan
Abstract
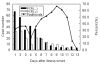
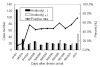
Severe acute respiratory syndrome (SARS) has raised a global alert since March 2003. After its causative agent, SARS-associated coronavirus (SARS-CoV), was confirmed, laboratory methods, including virus isolation, reverse transcriptase-polymerase chain reaction (RT-PCR), and serologic methods, have been quickly developed. In this study, we evaluated four serologic tests ( neutralization test, enzyme-linked immunosorbent assay [ELISA], immunofluorescent assay [IFA], and immunochromatographic test [ICT]) for detecting antibodies to SARS-CoV in sera of 537 probable SARS case-patients with correlation to the RT-PCR. With the neutralization test as a reference method, the sensitivity, specificity, positive predictive value, and negative predictive value were 98.2%, 98.7%, 98.7%, and 98.4% for ELISA; 99.1%, 87.8%, 88.1% and 99.1% for IFA; 33.6%, 98.2%, 95.7%, and 56.1% for ICT, respectively. We also compared the recombinant-based western blot with the whole virus-based IFA and ELISA; the data showed a high correlation between these methods, with an overall agreement of >90%. Our results provide a systematic analysis of serologic and molecular methods for evaluating SARS-CoV infection.
Figures
References
-
- CDC SARS Investigative Team. Fleischauer AT, Outbreak of severe acute respiratory syndrome—worldwide. MMWR Morb Mortal Wkly Rep. 2003;52:226–8. - PubMed
-
- Cumulative number of SARS probable cases. WHO. [Accessed July 3, 2003] Available from: URL: http://www.who.int/csr/sars/country/2003_06_25 /en/.
-
- Cumulative number of SARS probable cases in Taiwan, SARS online information center, Center for Disease Control, Taiwan. [Accessed August 2, 2003] Available from: URL: http://www.cdc.gov.tw/sarsen.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
