A nonsymbiotic root hair tip growth phenotype in NORK-mutated legumes: implications for nodulation factor-induced signaling and formation of a multifaceted root hair pocket for bacteria
- PMID: 15031407
- PMCID: PMC412867
- DOI: 10.1105/tpc.019653
A nonsymbiotic root hair tip growth phenotype in NORK-mutated legumes: implications for nodulation factor-induced signaling and formation of a multifaceted root hair pocket for bacteria
Abstract
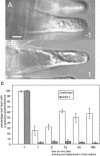
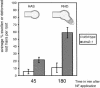
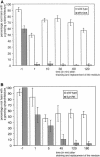
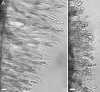
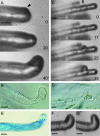
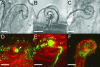
The Medicago truncatula Does not Make Infections (DMI2) mutant is mutated in the nodulation receptor-like kinase, NORK. Here, we report that NORK-mutated legumes of three species show an enhanced touch response to experimental handling, which results in a nonsymbiotic root hair phenotype. When care is taken not to induce this response, DMI2 root hairs respond morphologically like the wild type to nodulation factor (NF). Global NF application results in root hair deformation, and NF spot application induces root hair reorientation or branching, depending on the position of application. In the presence of Sinorhizobium meliloti, DMI2 root hairs make two-dimensional 180 degrees curls but do not entrap bacteria in a three-dimensional pocket because curling stops when the root hair tip touches its own shank. Because DMI2 does not express the promoter of M. truncatula Early Nodulin11 (ENOD11) coupled to beta-glucuronidase upon NF application, we propose a split in NF-induced signaling, with one branch to root hair curling and the other to ENOD11 expression.
Figures




Similar articles
-
The small GTPase ROP10 of Medicago truncatula is required for both tip growth of root hairs and nod factor-induced root hair deformation.Plant Cell. 2015 Mar;27(3):806-22. doi: 10.1105/tpc.114.135210. Epub 2015 Mar 20. Plant Cell. 2015. PMID: 25794934 Free PMC article.
-
Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula.Proc Natl Acad Sci U S A. 2000 Nov 21;97(24):13407-12. doi: 10.1073/pnas.230439797. Proc Natl Acad Sci U S A. 2000. PMID: 11078514 Free PMC article.
-
A transient decrease in reactive oxygen species in roots leads to root hair deformation in the legume-rhizobia symbiosis.New Phytol. 2007;173(1):39-49. doi: 10.1111/j.1469-8137.2006.01901.x. New Phytol. 2007. PMID: 17176392
-
Nitrate transporters: an overview in legumes.Planta. 2017 Oct;246(4):585-595. doi: 10.1007/s00425-017-2724-6. Epub 2017 Jun 26. Planta. 2017. PMID: 28653185 Review.
-
Leguminous plants: inventors of root nodules to accommodate symbiotic bacteria.Int Rev Cell Mol Biol. 2015;316:111-58. doi: 10.1016/bs.ircmb.2015.01.004. Epub 2015 Feb 20. Int Rev Cell Mol Biol. 2015. PMID: 25805123 Review.
Cited by
-
Symbiotic rhizobia bacteria trigger a change in localization and dynamics of the Medicago truncatula receptor kinase LYK3.Plant Cell. 2011 Jul;23(7):2774-87. doi: 10.1105/tpc.111.086389. Epub 2011 Jul 8. Plant Cell. 2011. PMID: 21742993 Free PMC article.
-
One-step Agrobacterium mediated transformation of eight genes essential for rhizobium symbiotic signaling using the novel binary vector system pHUGE.PLoS One. 2012;7(10):e47885. doi: 10.1371/journal.pone.0047885. Epub 2012 Oct 24. PLoS One. 2012. PMID: 23112864 Free PMC article.
-
The small GTPase ROP10 of Medicago truncatula is required for both tip growth of root hairs and nod factor-induced root hair deformation.Plant Cell. 2015 Mar;27(3):806-22. doi: 10.1105/tpc.114.135210. Epub 2015 Mar 20. Plant Cell. 2015. PMID: 25794934 Free PMC article.
-
How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model.Nat Rev Microbiol. 2007 Aug;5(8):619-33. doi: 10.1038/nrmicro1705. Nat Rev Microbiol. 2007. PMID: 17632573 Free PMC article. Review.
-
A small GTPase of the Rab family is required for root hair formation and preinfection stages of the common bean-Rhizobium symbiotic association.Plant Cell. 2009 Sep;21(9):2797-810. doi: 10.1105/tpc.108.063420. Epub 2009 Sep 11. Plant Cell. 2009. PMID: 19749154 Free PMC article.
References
-
- Ardourel, M., Demont, N., Debellé, F., Maillet, F., De Billy, F., Promé, J.-C., Dénarié, J., and Truchet, G. (1994). Rhizobium meliloti lipooligosaccharide nodulation factors: Different structural requirements for bacterial entry into target root hair cells and induction of plant symbiotic developmental responses. Plant Cell 6, 1357–1374. - PMC - PubMed
-
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C.R. Acad. Sci. III Vie 316, 1194–1199.
-
- Ben Amor, B., Shaw, S.L., Oldroyd, G.E.D., Maillet, F., Penmetsa, R.V., Cook, D., Long, S.R., Dénarié, J., and Gough, C. (2003). The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J. 34, 495–506. - PubMed
-
- Cárdenas, L., Feijó, J.A., Kunkel, J.G., Sánchez, F., Holdaway-Clarke, T., Hepler, P.K., and Quinto, C. (1999). Rhizobium Nod factors induce increases in intracellular free calcium influxes in bean root hairs. Plant J. 19, 347–352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

