Suppression of nuclear oscillations in Saccharomyces cerevisiae expressing Glu tubulin
- PMID: 15031428
- PMCID: PMC397425
- DOI: 10.1073/pnas.0307917101
Suppression of nuclear oscillations in Saccharomyces cerevisiae expressing Glu tubulin
Abstract
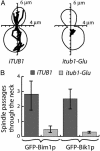
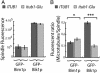
In most eukaryotic cells, the C-terminal amino acid of alpha-tubulin is aromatic (Tyr in mammals and Phe in Saccharomyces cerevisiae) and is preceded by two glutamate residues. In mammals, the C-terminal Tyr of alpha-tubulin is subject to cyclic removal from the peptide chain by a carboxypeptidase and readdition to the chain by a tubulin-Tyr ligase. There is evidence that tubulin-Tyr ligase suppression and the resulting accumulation of detyrosinated (Glu) tubulin favor tumor growth, both in animal models and in human cancers. However, the molecular basis for this apparent stimulatory effect of Glu tubulin accumulation on tumor progression is unknown. Here we have developed S. cerevisiae strains expressing only Glu tubulin and used them as a model to assess the consequences of Glu tubulin accumulation in cells. We find that Glu tubulin strains show defects in nuclear oscillations. These defects are linked to a markedly decreased association of the yeast ortholog of CLIP170, Bik1p, with microtubule plus-ends. These results indicate that the accumulation of Glu tubulin in cells affects microtubule tip complexes that are important for microtubule interactions with the cell cortex.
Figures




Comment in
-
Microtubule composition: cryptography of dynamic polymers.Proc Natl Acad Sci U S A. 2004 May 4;101(18):6839-40. doi: 10.1073/pnas.0401266101. Epub 2004 Apr 27. Proc Natl Acad Sci U S A. 2004. PMID: 15123818 Free PMC article. No abstract available.
References
-
- Lafanechere, L., Courtay-Cahen, C., Kawakami, T., Jacrot, M., Rudiger, M., Wehland, J., Job, D. & Margolis, R. L. (1998) J. Cell Sci. 111, 171–181. - PubMed
-
- Mialhe, A., Lafanechere, L., Treilleux, I., Peloux, N., Dumontet, C., Bremond, A., Panh, M. H., Payan, R., Wehland, J., Margolis, R. L. & Job, D. (2001) Cancer Res. 61, 5024–5027. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

