Cellular autophagy: surrender, avoidance and subversion by microorganisms
- PMID: 15031729
- PMCID: PMC7097095
- DOI: 10.1038/nrmicro865
Cellular autophagy: surrender, avoidance and subversion by microorganisms
Abstract
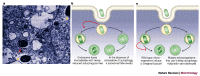
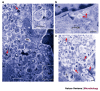
Intracellular bacteria and viruses must survive the vigorous antimicrobial responses of their hosts to replicate successfully. The cellular process of autophagy — in which compartments bound by double membranes engulf portions of the cytosol and then mature to degrade their cytoplasmic contents — is likely to be one such host-cell response. Several lines of evidence show that both bacteria and viruses are vulnerable to autophagic destruction and that successful pathogens have evolved strategies to avoid autophagy, or to actively subvert its components, to promote their own replication. The molecular mechanisms of the avoidance and subversion of autophagy by microorganisms will be the subject of much future research, not only to study their roles in the replication of these microorganisms, but also because they will provide — as bacteria and viruses so often have — unique tools to study the cellular process itself.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

