Gap junction channel gating
- PMID: 15033578
- PMCID: PMC2813678
- DOI: 10.1016/j.bbamem.2004.01.008
Gap junction channel gating
Abstract
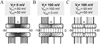
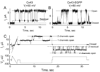
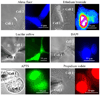
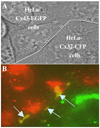
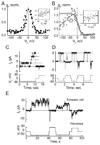
Over the last two decades, the view of gap junction (GJ) channel gating has changed from one with GJs having a single transjunctional voltage-sensitive (V(j)-sensitive) gating mechanism to one with each hemichannel of a formed GJ channel, as well as unapposed hemichannels, containing two, molecularly distinct gating mechanisms. These mechanisms are termed fast gating and slow or 'loop' gating. It appears that the fast gating mechanism is solely sensitive to V(j) and induces fast gating transitions between the open state and a particular substate, termed the residual conductance state. The slow gating mechanism is also sensitive to V(j), but there is evidence that this gate may mediate gating by transmembrane voltage (V(m)), intracellular Ca(2+) and pH, chemical uncouplers and GJ channel opening during de novo channel formation. A distinguishing feature of the slow gate is that the gating transitions appear to be slow, consisting of a series of transient substates en route to opening and closing. Published reports suggest that both sensorial and gating elements of the fast gating mechanism are formed by transmembrane and cytoplamic components of connexins among which the N terminus is most essential and which determines gating polarity. We propose that the gating element of the slow gating mechanism is located closer to the central region of the channel pore and serves as a 'common' gate linked to several sensing elements that are responsive to different factors and located in different regions of the channel.
Figures








References
-
- Bennett MV. Connexins in disease [news] Nature. 1994;368:18–19. - PubMed
-
- Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996;65:475–502. - PubMed
-
- Hertzberg EL, Disher RM, Tiller AA, Zhou Y, Cook RG. Topology of the Mr 27,000 liver gap junction protein. Cytoplasmic localization of amino- and carboxyl termini and a hydrophilic domain which is protease-hypersensitive. J. Biol. Chem. 1988;263:19105–19111. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

