Excitability of the T-tubular system in rat skeletal muscle: roles of K+ and Na+ gradients and Na+-K+ pump activity
- PMID: 15034125
- PMCID: PMC1665049
- DOI: 10.1113/jphysiol.2003.059014
Excitability of the T-tubular system in rat skeletal muscle: roles of K+ and Na+ gradients and Na+-K+ pump activity
Abstract
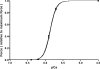
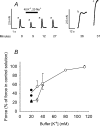
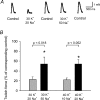
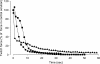
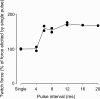
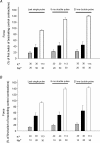
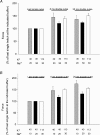
Strenuous exercise causes an increase in extracellular [K(+)] and intracellular Na(+) ([Na(+)](i)) of working muscles, which may reduce sarcolemma excitability. The excitability of the sarcolemma is, however, to some extent protected by a concomitant increase in the activity of muscle Na(+)-K(+) pumps. The exercise-induced build-up of extracellular K(+) is most likely larger in the T-tubules than in the interstitium but the significance of the cation shifts and Na(+)-K(+) pump for the excitability of the T-tubular membrane and the voltage sensors is largely unknown. Using mechanically skinned fibres, we here study the role of the Na(+)-K(+) pump in maintaining T-tubular function in fibres with reduced chemical K(+) gradient. The Na(+)-K(+) pump activity was manipulated by changing [Na(+)](i). The responsiveness of the T-tubules was evaluated from the excitation-induced force production of the fibres. Compared to control twitch force in fibres with a close to normal intracellular [K(+)] ([K(+)](i)), a reduction in [K(+)](i) to below 60 mM significantly reduced twitch force. Between 10 and 50 mM Na(+), the reduction in force depended on [Na(+)](i), the twitch force at 40 mM K(+) being 22 +/- 4 and 54 +/- 9% (of control force) at a [Na(+)](i) of 10 and 20 mM, respectively (n= 4). Double pulse stimulation of fibres at low [K(+)](i) showed that although elevated [Na(+)](i) increased the responsiveness to single action potentials, it reduced the capacity of the T-tubules to respond to high frequency stimulation. It is concluded that a reduction in the chemical gradient for K(+), as takes place during intensive exercise, may depress T-tubular function, but that a concomitant exercise-induced increase in [Na(+)](i) protects T-tubular function by stimulating the Na(+)-K(+) pump.
Figures

References
-
- Cairns SP, Buler SJ, Loiselle DS, Renaud JM. Changes of action potentials and force at lowered [Na+]o in mouse skeletal muscle: implications for fatigue. Am J Physiol. 2003;285:C1131–C1141. - PubMed
-
- Cairns SP, Flatman JA, Clausen T. Relation between extracellular [K+], membrane potential and contraction in rat soleus muscle: modulation by the Na+–K+ pump. Pflugers Arch. 1995;430:909–915. - PubMed
-
- Cairns SP, Hing WA, Slack JR, Mills RG, Loiselle DS. Different effects of raised [K+]o on membrane potential and contraction in mouse fast- and slow-twitch muscle. Am J Physiol. 1997;273:C598–C611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

