BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus
- PMID: 15034127
- PMCID: PMC1665041
- DOI: 10.1113/jphysiol.2004.062661
BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus
Abstract
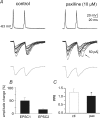
Large conductance calcium- and voltage-activated potassium channels (BK channels) activate in response to calcium influx during action potentials and contribute to the spike repolarization and fast afterhyperpolarization. BK channels targeted to active zones in presynaptic nerve terminals have been shown to limit calcium entry and transmitter release by reducing the duration of the presynaptic spike at neurosecretory nerve terminals and at the frog neuromuscular junction. However, their functional role in central synapses is still uncertain. In the hippocampus, BK channels have been proposed to act as an 'emergency brake' that would control transmitter release only under conditions of excessive depolarization and accumulation of intracellular calcium. Here we demonstrate that in the CA3 region of hippocampal slice cultures, under basal experimental conditions, the selective BK channel blockers paxilline (10 microM) and iberiotoxin (100 nM) increase the frequency, but not the amplitude, of spontaneously occurring action potential-dependent EPSCs. These drugs did not affect miniature currents recorded in the presence of tetrodotoxin, suggesting that their action was dependent on action potential firing. Moreover, in double patch-clamp recordings from monosynaptically interconnected CA3 pyramidal neurones, blockade of BK channels enhanced the probability of transmitter release, as revealed by the increase in success rate, EPSC amplitude and the concomitant decrease in paired-pulse ratio in response to pairs of presynaptic action potentials delivered at a frequency of 0.05 Hz. BK channel blockers also enhanced the appearance of delayed responses, particularly following the second action potential in the paired-pulse protocol. These results are consistent with the hypothesis that BK channels are powerful modulators of transmitter release and synaptic efficacy in central neurones.
Figures





References
-
- Adams PR, Constanti A, Brown DA, Clark RB. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982;296:746–749. - PubMed
-
- Blundon JA, Wright SN, Brodwick MS, Bittner GD. Presynaptic calcium-activated potassium channels and calcium channels at a crayfish neuromuscular junction. J Neurophysiol. 1995;73:178–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

