A point mutation in the motor domain of nonmuscle myosin II-B impairs migration of distinct groups of neurons
- PMID: 15034141
- PMCID: PMC420083
- DOI: 10.1091/mbc.e03-11-0836
A point mutation in the motor domain of nonmuscle myosin II-B impairs migration of distinct groups of neurons
Abstract
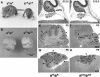
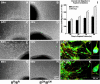
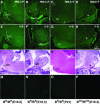
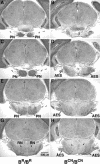
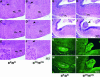
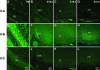
We generated mice harboring a single amino acid mutation in the motor domain of nonmuscle myosin heavy chain II-B (NMHC II-B). Homozygous mutant mice had an abnormal gait and difficulties in maintaining balance. Consistent with their motor defects, the mutant mice displayed an abnormal pattern of cerebellar foliation. Analysis of the brains of homozygous mutant mice showed significant defects in neuronal migration involving granule cells in the cerebellum, the facial neurons, and the anterior extramural precerebellar migratory stream, including the pontine neurons. A high level of NMHC II-B expression in these neurons suggests an important role for this particular isoform during neuronal migration in the developing brain. Increased phosphorylation of the myosin II regulatory light chain in migrating, compared with stationary pontine neurons, supports an active role for myosin II in regulating their migration. These studies demonstrate that NMHC II-B is particularly important for normal migration of distinct groups of neurons during mouse brain development.
Figures



References
-
- Altman, J., and Bayer, S.A. (1987). Development of the precerebellar nuclei in the rat. J. Comp. Neurol. 257, 477-552. - PubMed
-
- Altman, J., and Bayer, S.A. (1997). Development of the cerebellar system in relation to its evolution, structure, and functions, Boca Raton, FL: CRC Press.
-
- Brown, M.E., and Bridgman, P.C. (2003). Retrograde flow is increased in growth cones from myosin IIB knockout mice. J. Cell Sci. 116, 1087-1094. - PubMed
-
- Buxton, D.B., Golomb, E., and Adelstein, R.S. (2003). Induction of nonmuscle myosin heavy chain II-C by butyrate in RAW 264.7 mouse macrophages. J. Biol. Chem. 278, 15449-15455. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

