The major form of MeCP2 has a novel N-terminus generated by alternative splicing
- PMID: 15034150
- PMCID: PMC390342
- DOI: 10.1093/nar/gkh349
The major form of MeCP2 has a novel N-terminus generated by alternative splicing
Abstract
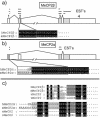
MeCP2 is a methyl-CpG binding protein that can repress transcription of nearby genes. In humans, mutations in the MECP2 gene are the major cause of Rett syndrome. By searching expressed sequence tag (EST) databases we have found a novel MeCP2 splice isoform (MeCP2alpha) which encodes a distinct N-terminus. We demonstrate that the MeCP2alpha mRNA splice variant is more abundant than the previously annotated MeCP2 mRNA (MeCP2beta) in mouse tissues and human brain. Furthermore, MeCP2beta mRNA has an upstream open reading frame that inhibits its translation. As a result of these differences, >90% of MeCP2 in mouse brain is MeCP2alpha. Both protein isoforms are nuclear and colocalize with densely methylated heterochromatic foci in mouse cells. The presence of a previously unknown MeCP2 isoform has implications for the genetic screening of Rett syndrome patients and for studies of the functional significance of MeCP2.
Figures



References
-
- El Osta A. and Wolffe,A.P. (2001) Analysis of chromatin-immunopurified MeCP2-associated fragments. Biochem. Biophys. Res. Commun., 289, 733–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

