Low temperature method for the production of calcium phosphate fillers
- PMID: 15035671
- PMCID: PMC406414
- DOI: 10.1186/1475-925X-3-8
Low temperature method for the production of calcium phosphate fillers
Abstract
Background: Calcium phosphate manufactured samples, prepared with hydroxyapatite, are used as either spacers or fillers in orthopedic surgery, but these implants have never been used under conditions of mechanical stress. Similar conditions also apply with cements. Many authors have postulated that cements are a useful substitute material when implanted in vivo. The aim of this research is to develop a low cristalline material similar to bone in porosity and cristallinity.
Methods: Commercial hydroxyapatite (HAp) and monetite (M) powders are mixed with water and compacted to produce cylindrical samples. The material is processed at a temperature of 37-120 degrees C in saturated steam to obtain samples that are osteoconductive. The samples are studied by X-ray powder diffraction (XRD), Vickers hardness test (HV), scanning electron microscopy (SEM), and porosity evaluation.
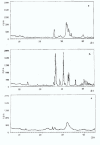
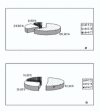
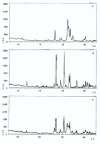
Results: The X-ray diffractions of powders from the samples show patterns typical of HAp and M powders. After thermal treatment, no new crystal phase is formed and no increase of the relative intensity of the peaks is obtained. Vicker hardness data do not show any relationship with treatment temperature. The total porosity decreases by 50-60% according to the specific thermal treatment. Scanning electron microscopy of the surfaces of the samples with either HAp 80%-M 20% (c) or Hap 50%-M 50% (f), show cohesion of the powder grains.
Conclusions: The dissolution-reprecipitation process is more intesive in manufactured samples (c) and (f), according to Vickers hardness data. The process occurs in a steam saturated environment between 37 degrees and 120 degrees C. (c) (f) manufactured samples show pore dimension distributions useful to cellular repopulation in living tissues.
Figures







References
-
- Daculsi G, Passutí N, Martin S, Deudon S, LeGeros RE, Rather S. Macroporous calcium phosphate ceramic for long bone surgery in humans and dogs. Clinical and histological study. J Biomed Mater Res. 1990;24:379–396. - PubMed
-
- Suh H, Lee C. Biodegradable ceramic-collagen composite implanted in rabbit tibiae. Asaio J. 1995;41:M652–656. - PubMed
-
- Jarcho M. Retrospective analysis of hydroxyapatite development for oral implant applications. Dent Clin North Am. 1992;36:19–26. - PubMed
-
- Capanna R, Manfrini M, Tigani D, Giunti A. Tricalcium phosphate and hydroxyapatite ceramics in the surgery of bone tumors: preliminary results. Chir Organi Mov. 1991;76:245–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

