Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis
- PMID: 15037444
- PMCID: PMC1755042
- DOI: 10.1136/ard.2003.016386
Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis
Abstract
Background: TNFalpha blockers have been shown to be highly efficacious in patients with active ankylosing spondylitis (AS).
Objective: To identify parameters predicting the clinical response to TNF blockers in AS.
Methods: Patients with active AS participated in two placebo controlled, randomised trials conducted in Germany with infliximab (n = 69) and etanercept (n = 30), respectively. For inclusion in either trial patients had to have high disease activity (BASDAI >or=4) and a spinal pain score (numerical rating scale 0-10) >or=4 despite treatment with NSAIDs. A major clinical response was defined as a 50% improvement of the initial BASDAI (BASDAI 50) after 12 weeks' treatment with active drug. Logistic regression likelihood ratio tests (univariate and multivariate), Student's t test, and chi(2) tests were performed.
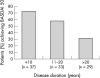
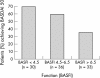
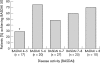
Results: Univariate analysis showed the following to be predictors of a major response (BASDAI 50) to treatment: shorter disease duration (p = 0.003); lower BASFI (p = 0.007); younger age (p = 0.009); raised ESR (p = 0.033); raised CRP (p = 0.035). After adjustment for disease duration, BASFI, ESR, and CRP, but not age, remained significantly associated. After adjustment for disease duration and for BASFI, ESR, CRP, and in addition, a higher BASDAI were significantly associated with response. The best multivariate model built by stepwise regression contained the covariables disease duration, BASFI, BASDAI, and CRP.
Conclusion: A shorter disease duration, younger age, and a lower BASFI are predictors of a major clinical response to TNF blockers in active AS. Raised CRP and a higher BASDAI may also be valuable predictors. These data need to be confirmed in further studies.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

