YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta
- PMID: 15037601
- PMCID: PMC2172068
- DOI: 10.1083/jcb.200310061
YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta
Abstract
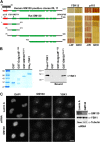
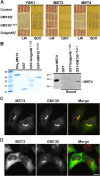
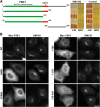
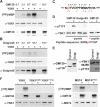
The Golgi apparatus has long been suggested to be important for directing secretion to specific sites on the plasma membrane in response to extracellular signaling events. However, the mechanisms by which signaling events are coordinated with Golgi apparatus function remain poorly understood. Here, we identify a scaffolding function for the Golgi matrix protein GM130 that sheds light on how such signaling events may be regulated. We show that the mammalian Ste20 kinases YSK1 and MST4 target to the Golgi apparatus via the Golgi matrix protein GM130. In addition, GM130 binding activates these kinases by promoting autophosphorylation of a conserved threonine within the T-loop. Interference with YSK1 function perturbs perinuclear Golgi organization, cell migration, and invasion into type I collagen. A biochemical screen identifies 14-3-3zeta as a specific substrate for YSK1 that localizes to the Golgi apparatus, and potentially links YSK1 signaling at the Golgi apparatus with protein transport events, cell adhesion, and polarity complexes important for cell migration.
Figures





Similar articles
-
YSK1, a novel mammalian protein kinase structurally related to Ste20 and SPS1, but is not involved in the known MAPK pathways.Oncogene. 1997 May 1;14(17):2047-57. doi: 10.1038/sj.onc.1201043. Oncogene. 1997. PMID: 9160885
-
CCM3/PDCD10 stabilizes GCKIII proteins to promote Golgi assembly and cell orientation.J Cell Sci. 2010 Apr 15;123(Pt 8):1274-84. doi: 10.1242/jcs.061341. Epub 2010 Mar 23. J Cell Sci. 2010. PMID: 20332113
-
Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis.Cell. 1998 Sep 18;94(6):783-93. doi: 10.1016/s0092-8674(00)81737-7. Cell. 1998. PMID: 9753325
-
Emerging new roles of GM130, a cis-Golgi matrix protein, in higher order cell functions.J Pharmacol Sci. 2010;112(3):255-64. doi: 10.1254/jphs.09r03cr. Epub 2010 Mar 2. J Pharmacol Sci. 2010. PMID: 20197635 Review.
-
Cell motility: Golgi signalling shapes up to ship out.Curr Biol. 2004 Jun 8;14(11):R434-5. doi: 10.1016/j.cub.2004.05.038. Curr Biol. 2004. PMID: 15182693 Review.
Cited by
-
Astrocytic neogenin/netrin-1 pathway promotes blood vessel homeostasis and function in mouse cortex.J Clin Invest. 2020 Dec 1;130(12):6490-6509. doi: 10.1172/JCI132372. J Clin Invest. 2020. PMID: 32853179 Free PMC article.
-
Focal defects in single-celled tubes mutant for Cerebral cavernous malformation 3, GCKIII, or NSF2.Dev Cell. 2013 Jun 10;25(5):507-19. doi: 10.1016/j.devcel.2013.05.002. Dev Cell. 2013. PMID: 23763949 Free PMC article.
-
Onco-Golgi: Is Fragmentation a Gate to Cancer Progression?Biochem Mol Biol J. 2015;1(1):16. doi: 10.21767/2471-8084.100006. Epub 2015 Nov 7. Biochem Mol Biol J. 2015. PMID: 27064441 Free PMC article.
-
C-Jun N-terminal kinase (JNK) pathway activation is essential for dental papilla cells polarization.PLoS One. 2021 Mar 26;16(3):e0233944. doi: 10.1371/journal.pone.0233944. eCollection 2021. PLoS One. 2021. PMID: 33770099 Free PMC article.
-
MAPK signaling to the early secretory pathway revealed by kinase/phosphatase functional screening.J Cell Biol. 2010 Jun 14;189(6):997-1011. doi: 10.1083/jcb.200912082. J Cell Biol. 2010. PMID: 20548102 Free PMC article.
References
-
- Barr, F.A., and B. Short. 2003. Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 15:405–413. - PubMed
-
- Barr, F.A., M. Puype, J. Vandekerckhove, and G. Warren. 1997. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 91:253–262. - PubMed
-
- Benton, R., I.M. Palacios, and D. St Johnston. 2002. Drosophila 14-3-3/PAR-5 is an essential mediator of PAR-1 function in axis formation. Dev. Cell. 3:659–671. - PubMed
-
- Bialkowska, K., Y. Zaffran, S.C. Meyer, and J.E. Fox. 2003. 14-3-3 zeta mediates integrin-induced activation of Cdc42 and Rac. Platelet glycoprotein Ib-IX regulates integrin-induced signaling by sequestering 14-3-3 zeta. J. Biol. Chem. 278:33342–33350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

