The CtBP2 co-repressor is regulated by NADH-dependent dimerization and possesses a novel N-terminal repression domain
- PMID: 15037661
- PMCID: PMC390340
- DOI: 10.1093/nar/gkh344
The CtBP2 co-repressor is regulated by NADH-dependent dimerization and possesses a novel N-terminal repression domain
Abstract
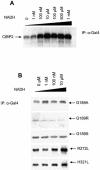
The C-terminal binding protein 2 (CtBP2) is a 48 kDa phosphoprotein reported to function as a co- repressor for a growing list of transcriptional repressors. It was recently demonstrated that CtBP is a dimeric NAD+-regulated d-isomer-specific 2-hydroxy acid dehydrogenase. However, the specific substrate(s) of CtBP enzymatic activity and the relationship of this activity to its co-repression function remain unknown. The ability of a human CtBP to bind and serve as a co-repressor of E1A has been shown to be regulated by nuclear NADH levels. Here we extend the functional characterization of CtBP by demonstrating that amino acid substitutions at Gly189 in the conserved NAD+-binding fold both abrogate the ability of CtBP2 to homodimerize and are associated with a dramatic loss of co-repressor activity. Consistent with the known enzymatic activity of CtBP2, mutations at Arg272 in the substrate-binding domain and at His321 in the catalytic domain result in significant loss of CtBP2 transcriptional co-repressor activity. High resolution serial C-terminal deletion analysis of CtBP2 also revealed a novel N-terminal repression domain that is distinct from its dehydrogenase domain. Our results suggest a model in which CtBP2 co-repressor function is regulated, at least in part, through the effect of NADH on CtBP2 homodimerization.
Figures





References
-
- Turner J. and Crossley,M. (2001) The CtBP family: enigmatic and enzymatic transcriptional co-repressors. Bioessays, 23, 683–690. - PubMed
-
- Chinnadurai G. (2002) CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol. Cell, 9, 213–224. - PubMed
-
- Boyd J.M., Subramanian,T., Scheaper,U., La Regina,M., Bayley,S. and Chinnadurai,G. (1993) A region in the C-terminus of adenovirus 2/5 E1A protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis and metastasis. EMBO J., 12, 469–478. - PMC - PubMed
-
- Scheaper U., Boyd,J.M., Verma,S., Uhlmann,E., Subramanian,T. and Chinnadurai,G. (1995) Molecular cloning and characterization of a cellular phosphoprotein that interacts with a conserved C-terminal domain of adenovirus E1A involved in negative modulation of oncogenic transformation. Proc. Natl Acad. Sci. USA, 92, 10467–10471. - PMC - PubMed
-
- Subramanian T., La Regina,M. and Chinnadurai,G. (1989) Enhanced ras oncogene mediated cell transformation and tumorigenesis by adenovirus 2 mutants lacking the C-terminal region of E1A protein. Oncogene, 4, 415–420. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

