The extracellular matrix, p53 and estrogen compete to regulate cell-surface Fas/Apo-1 suicide receptor expression in proliferating embryonic cerebral cortical precursors, and reciprocally, Fas-ligand modifies estrogen control of cell-cycle proteins
- PMID: 15038834
- PMCID: PMC395829
- DOI: 10.1186/1471-2202-5-11
The extracellular matrix, p53 and estrogen compete to regulate cell-surface Fas/Apo-1 suicide receptor expression in proliferating embryonic cerebral cortical precursors, and reciprocally, Fas-ligand modifies estrogen control of cell-cycle proteins
Abstract
Background: Apoptosis is important for normal cerebral cortical development. We previously showed that the Fas suicide receptor was expressed within the developing cerebral cortex, and that in vitro Fas activation resulted in caspase-dependent death. Alterations in cell-surface Fas expression may significantly influence cortical development. Therefore, in the following studies, we sought to identify developmentally relevant cell biological processes that regulate cell-surface Fas expression and reciprocal consequences of Fas receptor activation.
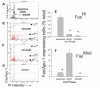
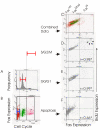
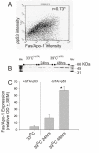
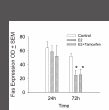
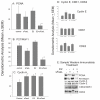
Results: Flow-cytometric analyses identified two distinct neural sub-populations that expressed Fas on their cell surface at high (FasHi) or moderate (FasMod) levels. The anti-apoptotic protein FLIP further delineated a subset of Fas-expressing cells with potential apoptosis-resistance. FasMod precursors were mainly in G0, while FasHi precursors were largely apoptotic. However, birth-date analysis indicated that neuroblasts express the highest levels of cell-surface Fas at the end of S-phase, or after their final round of mitosis, suggesting that Fas expression is induced at cell cycle checkpoints or during interkinetic nuclear movements. FasHi expression was associated with loss of cell-matrix adhesion and anoikis. Activation of the transcription factor p53 was associated with induction of Fas expression, while the gonadal hormone estrogen antagonistically suppressed cell-surface Fas expression. Estrogen also induced entry into S-phase and decreased the number of Fas-expressing neuroblasts that were apoptotic. Concurrent exposure to estrogen and to soluble Fas-ligand (sFasL) suppressed p21/waf-1 and PCNA. In contrast, estrogen and sFasL, individually and together, induced cyclin-A expression, suggesting activation of compensatory survival mechanisms.
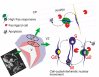
Conclusions: Embryonic cortical neuronal precursors are intrinsically heterogeneous with respect to Fas suicide-sensitivity. Competing intrinsic (p53, cell cycle, FLIP expression), proximal (extra-cellular matrix) and extrinsic factors (gonadal hormones) collectively regulate Fas suicide-sensitivity either during neurogenesis, or possibly during neuronal migration, and may ultimately determine which neuroblasts successfully contribute neurons to the differentiating cortical plate.
Figures


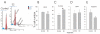
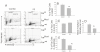
References
-
- Finlay B, Slattery M. Local differences in the amount of early cell death in neocortex predict adult local specializations. Science. 1983;219:1349–1351. - PubMed
-
- Spreafico R, Frassoni C, Arcelli P, Selvaggio M, De Biasi S. In situ labeling of apoptotic cell death in the cerebral cortex and thalamus of rats during development. J Comp Neurol. 1995;363:281–295. - PubMed
-
- Rabinowicz T, De Courten-Myers GM, Petetot JM-S, Xi G, De Los Reyes E. Human cortex development: Estimates of neuronal numbers indicate major loss late during gestation. Journal of Neuropathology and Experimental Neurology. 1996;55:320–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

