Mechanisms involved in apoptosis of human macrophages induced by lipopolysaccharide from Actinobacillus actinomycetemcomitans in the presence of cycloheximide
- PMID: 15039304
- PMCID: PMC375163
- DOI: 10.1128/IAI.72.4.1856-1865.2004
Mechanisms involved in apoptosis of human macrophages induced by lipopolysaccharide from Actinobacillus actinomycetemcomitans in the presence of cycloheximide
Abstract
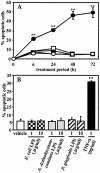
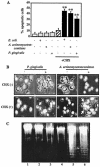
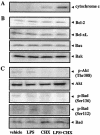
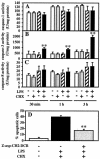
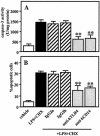
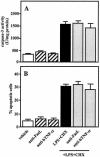
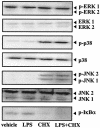
Actinobacillus actinomycetemcomitans is a major periodontopathic bacterium with multiple virulence factors, including lipopolysaccharide (LPS). Previous reports have demonstrated that LPS induced apoptosis in a murine macrophage-like cell line, J744.1, as well as in peritoneal macrophages from C3H/HeN mice in the presence of cycloheximide (CHX). However, the detailed molecular mechanisms involved in the apoptosis of macrophages induced by LPS and CHX are not well known. To clarify the possible role of LPS in the induction of macrophage apoptosis, we investigated cell death induced by LPS from A. actinomycetemcomitans and CHX in human macrophage-like U937 cells, which were differentiated by 12-O-tetradecanoylphorbol 13-acetate (TPA), and also assessed the molecular mechanisms involved in the process. We found that TPA-differentiated U937 cells usually showed resistance to LPS-induced apoptosis. However, in the presence of CHX, LPS induced release of cytochrome c without modifying steady-state levels of Bcl-2, Bcl-xL, Bax, and Bak. Treatment with LPS in the presence of CHX also led to activation of caspase-3 and apoptosis via, in part, the CD14/toll-like receptor 4 (TLR4). The induction of cytochrome c release may have been due to dephosphorylation of Akt and Bad, which were cooperatively induced by CHX and LPS. However, endogenous tumor necrosis factor alpha- and Fas-induced signals, extracellular signal-regulated kinase kinase/mitogen-activated protein kinases and I-kappa B alpha/nuclear factor-kappa B (NF-kappa B) were not required for caspase-3-dependent apoptosis. These results emphasize the possible important role of the mitochondrial apoptotic pathway leading to caspase-3 activation in LPS-induced apoptosis of human macrophages in the presence of CHX.
Figures

References
-
- Afford, S. C., J. Pongracz, R. A. Stockley, J. Crocker, and D. Burnett. 1992. The induction by human interleukin-6 of apoptosis in the promonocytic cell line U937 and human neutrophils. J. Biol. Chem. 267:21612-21616. - PubMed
-
- Amano, F., and H. Karahashi. 1996. A cytotoxic effect of lipopolysaccharide on a macrophage-like cell line, J774.1, in the presence of cycloheximide. J. Endotoxin Res. 3:415-423. - PubMed
-
- Bremner, T. A., D. Chatterjee, Z. Han, M. F. Tsan, and J. H. Wyche. 1999. THP-1 monocytic leukemia cells express Fas ligand constitutively and kill Fas-positive Jurkat cells. Leuk. Res. 23:865-870. - PubMed
-
- Choi, K. B., F. Wong, J. M. Harlan, P. M. Chaudhary, L. Hood, and A. Karsan. 1998. Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J. Biol. Chem. 273:20185-20188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

