N-linked glycosylation is required for c1 inhibitor-mediated protection from endotoxin shock in mice
- PMID: 15039314
- PMCID: PMC375168
- DOI: 10.1128/IAI.72.4.1946-1955.2004
N-linked glycosylation is required for c1 inhibitor-mediated protection from endotoxin shock in mice
Abstract
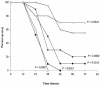
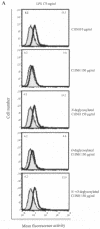
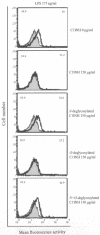
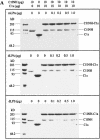
C1 inhibitor (C1INH) prevents endotoxin shock in mice via a direct interaction with lipopolysaccharide (LPS). This interaction requires the heavily glycosylated amino-terminal domain of C1INH. C1INH in which N-linked carbohydrate was removed by using N-glycosidase F was markedly less effective in protecting mice from LPS-induced lethal septic shock. N-deglycosylated C1INH also failed to suppress fluorescein isothiocyanate (FITC)-LPS binding to and LPS-induced tumor necrosis factor alpha mRNA expression by the murine macrophage-like cell line, RAW 264.7, and cells in human whole blood. In an enzyme linked immunosorbent assay, the N-deglycosylated C1INH bound to LPS very poorly. In addition, C1INH was shown to bind to diphosphoryl lipid A (dLPA) but only weakly to monophosphoryl lipid A (mLPA). As with intact LPS, binding of N-deglycosylated C1INH to dLPA and mLPA was diminished in comparison with the native protein. Removal of O-linked carbohydrate had no effect on any of these activities. Neither detoxified LPS, dLPA, nor mLPA had any effect on the rate or extent of C1INH complex formation with C1s or on cleavage of the reactive center loop by trypsin. These data demonstrate that N-linked glycosylation of C1INH is essential to mediate its interaction with the LPA moiety of LPS and to protect mice from endotoxin shock.
Figures









References
-
- Astiz, M. E., R. C. Rackow, J. G. Still, S. T. Howell, A. Cato, K. B. Von Eschen, J. T. Ulrich, J. A. Rudbach, G. McMahon, R. Vargas, and W. Stern. 1995. Pretreatment of normal humans with monophosphoryl lipid A induces tolerance to endotoxin: a prospective, double-blind, randomized, controlled trial. Crit. Care Med. 23:9-17. - PubMed
-
- Battafarano, R. J., P. S. Dahlberg, C. A. Ratz, J. W. Johnston, B. H. Gray, J. R. Haseman, K. H. Mayo, and D. L. Dunn. 1995. Peptide derivatives of three distinct lipopolysaccharide binding proteins inhibit lipopolysaccharide-induced tumor necrosis factor-alpha secretion in vitro. Surgery 118:318-324. - PubMed
-
- Bock, S. C., K. Skriver, E. Nielsen, H. C. Thogersen, B. Wiman, V. H. Donaldson, R. L. Eddy, J. Marrinan, E. Radziejewska, R. Huber, T. B. Shows, and S. Magnussen. 1986. Human C1 inhibitor: primary structure, cDNA cloning, and chromosomal localization. Biochemistry 25:4292-4301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

