Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages
- PMID: 15039328
- PMCID: PMC375177
- DOI: 10.1128/IAI.72.4.2067-2074.2004
Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages
Abstract
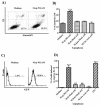
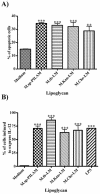
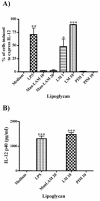
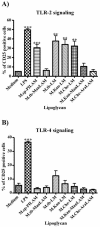
The mycobacterial cell wall component lipoarabinomannan (LAM) has been described as a virulence factor of Mycobacterium tuberculosis, and modification of the terminal arabinan residues of this compound with mannose caps (producing mannosyl-capped LAM [ManLAM]) in M. tuberculosis or with phosphoinositol caps (producing phosphoinositol-capped LAM [PILAM]) in Mycobacterium smegmatis has been implicated in various functions associated with these lipoglycans. A structure-function analysis was performed by using LAMs and their biosynthetic precursor lipomannans (LMs) isolated from different mycobacterial species on the basis of their capacity to induce the production of interleukin-12 (IL-12) and/or apoptosis of macrophage cell lines. Independent of the mycobacterial species, ManLAMs did not induce IL-12 gene expression or apoptosis of macrophages, whereas PILAMs induced IL-12 secretion and apoptosis. Interestingly, uncapped LAM purified from Mycobacterium chelonae did not induce IL-12 secretion or apoptosis. Furthermore, LMs, independent of their mycobacterial origins, were potent inducers of IL-12 and apoptosis. The precursor of LM, phosphatidyl-myo-inositol dimannoside, had no activity, suggesting that the mannan core of LM was required for the activity of LM. The specific interaction of LM with Toll-like receptor 2 (TLR-2) but not with TLR-4 suggested that these responses were mediated via the TLR-2 signaling pathway. Our experiments revealed an important immunostimulatory activity of the biosynthetic LAM precursor LM. The ratio of LAM to LM in the cell wall of mycobacteria may be an important determinant of virulence, and enzymes that modify LM could provide targets for development of antituberculosis drugs and for derivation of attenuated strains of M. tuberculosis.
Figures

References
-
- Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. - PubMed
-
- Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432-1435. - PubMed
-
- Brennan, P. J. 2003. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb.) 83:91-97. - PubMed
-
- Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

