Inhibition of major histocompatibility complex II expression and antigen processing in murine alveolar macrophages by Mycobacterium bovis BCG and the 19-kilodalton mycobacterial lipoprotein
- PMID: 15039332
- PMCID: PMC375182
- DOI: 10.1128/IAI.72.4.2101-2110.2004
Inhibition of major histocompatibility complex II expression and antigen processing in murine alveolar macrophages by Mycobacterium bovis BCG and the 19-kilodalton mycobacterial lipoprotein
Abstract
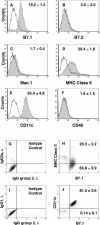
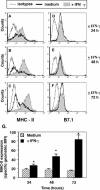
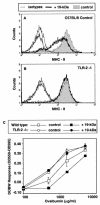
Alveolar macrophages constitute a primary defense against Mycobacterium tuberculosis, but they are unable to control M. tuberculosis without acquired T-cell immunity. This study determined the antigen-presenting cell function of murine alveolar macrophages and the ability of the model mycobacterium, Mycobacterium bovis BCG, to modulate it. The majority (80 to 85%) of alveolar macrophages expressed both CD80 (B7.1) and CD11c, and 20 to 30% coexpressed major histocompatibility complex II (MHC-II). Gamma interferon (IFN-gamma) enhanced MHC-II but not B7.1 expression. Naive or IFN-gamma-treated alveolar macrophages did not express CD86 (B7.2), CD11b, Mac-3, CD40, or F4/80. M. bovis BCG and the 19-kDa mycobacterial lipoprotein inhibited IFN-gamma-regulated MHC-II expression on alveolar macrophages, and inhibition was dependent on Toll-like receptor 2. The inhibition of MHC-II expression by the 19-kDa lipoprotein was associated with decreased presentation of soluble antigen to T cells. Thus, susceptibility to tuberculosis may result from the ability of mycobacteria to interfere with MHC-II expression and antigen presentation by alveolar macrophages.
Figures




References
-
- Beatty, W. L., E. R. Rhoades, H. J. Ullrich, D. Chatterjee, J. E. Heuser, and D. G. Russell. 2000. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 1:235-247. - PubMed
-
- Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. - PubMed
-
- Chensue, S. W., K. Warmington, J. Ruth, P. Lincoln, M. C. Kuo, and S. L. Kunkel. 1994. Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor. Am. J. Pathol. 145:1105-1113. - PMC - PubMed
-
- Constant, S. L., K. S. Lee, and K. Bottomly. 2000. Site of antigen delivery can influence T cell priming: pulmonary environment promotes preferential Th2-type differentiation. Eur. J. Immunol. 30:840-847. - PubMed
-
- Cooper, A. M., J. E. Callahan, M. Keen, J. T. Belisle, and I. M. Orme. 1997. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tuber. Lung Dis. 78:67-73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI-95383/AI/NIAID NIH HHS/United States
- K8-HL04299/HL/NHLBI NIH HHS/United States
- R01 AI035726/AI/NIAID NIH HHS/United States
- AI-27243/AI/NIAID NIH HHS/United States
- HL55967/HL/NHLBI NIH HHS/United States
- K08 HL004299/HL/NHLBI NIH HHS/United States
- AI-44794/AI/NIAID NIH HHS/United States
- R01 HL055967/HL/NHLBI NIH HHS/United States
- R01 AI034343/AI/NIAID NIH HHS/United States
- R01 AI027243/AI/NIAID NIH HHS/United States
- AI-35726/AI/NIAID NIH HHS/United States
- AI-36219/AI/NIAID NIH HHS/United States
- AI-34343/AI/NIAID NIH HHS/United States
- N01 AI095383/AI/NIAID NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

