Normally occurring human anti-GM1 immunoglobulin M antibodies and the immune response to bacteria
- PMID: 15039337
- PMCID: PMC375194
- DOI: 10.1128/IAI.72.4.2148-2151.2004
Normally occurring human anti-GM1 immunoglobulin M antibodies and the immune response to bacteria
Abstract
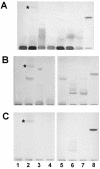
Anti-GM(1) antibodies of the immunoglobulin M (IgM) isotype are normal components of the antibody repertoire of adult human serum. Using a sensitive high-performance thin-layer chromatography (HPTLC) immunostaining assay, we found that these antibodies were absent in the umbilical vein and children <1 month of age but could be detected after 1 month of age. Although most of the children older than 6 months of age were positive, there were still a few negative children. The appearance of anti-GM(1) IgM antibodies showed a perfect concordance with two well-characterized antibacterial antibodies, anti-Forssman and anti-blood group A, which indicates a similar origin. We also studied IgM reactivity with lipopolysaccharides (LPSs) from gram-negative bacteria isolated from stool samples from healthy babies and from Escherichia coli HB101 in serum from individuals of different ages. We found a positive reaction with both LPSs in all the children more than 1 month of age analyzed, even in those that were negative for anti-GM(1) antibodies. Anti-GM(1) IgM antibodies were purified from adult serum by affinity chromatography and tested for the ability to bind LPSs from different bacteria. This highly specific preparation showed reactivity only with LPS from a strain of Campylobacter jejuni isolated from a patient with diarrhea. We conclude that normally occurring IgM antibodies are generated after birth, probably during the immune defense against specific bacterial strains.
Figures


References
-
- Arasaki, K., S. Kusoniki, N. Kudo, and I. Kanasawa. 1993. Acute conduction block in vitro following exposure to anti-ganglioside sera. Muscle Nerve 16:587-593. - PubMed
-
- Cumar, F. A., H. S. Barra, H. J. Maccioni, and R. Caputto. 1968. Sulfation of glycosphingolipids and related carbohydrates by brain preparation from young rats. J. Biol. Chem. 246:5075-5084. - PubMed
-
- Glass, R. I., B. J. Stoll, M. I. Huq, M. J. Struelens, M. Blaser, and A. K. Kibriya. 1983. Epidemiologic and clinical features of endemic Campylobacter jejuni infection in Bangladesh. J. Infect. Dis. 148:292-296. - PubMed
-
- Hirabayashi, Y., T. Susuki, Y. Susuki, T. Taki, M. Matsumoto, H. Higashi, and S. Kato. 1983. A new method for purification of anti-glycosphingolipid antibody. Avian anti-hematoside (NeuGc) antibody. J. Biochem. 94:327-330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

