Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7
- PMID: 15039348
- PMCID: PMC375161
- DOI: 10.1128/IAI.72.4.2240-2247.2004
Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7
Abstract
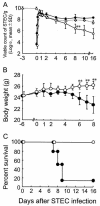
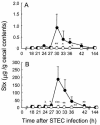
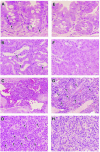
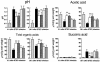
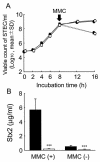
The anti-infectious activity of probiotic Bifidobacteria against Shiga toxin-producing Escherichia coli (STEC) O157:H7 was examined in a fatal mouse STEC infection model. Stable colonization of the murine intestines was achieved by the oral administration of Bifidobacterium breve strain Yakult (naturally resistant to streptomycin sulfate) as long as the mice were treated with streptomycin in their drinking water (5 mg/ml). The pathogenicity of STEC infection, characterized by marked body weight loss and subsequent death, observed in the infected controls was dramatically inhibited in the B. breve-colonized group. Moreover, Stx production by STEC cells in the intestine was almost completely inhibited in the B. breve-colonized group. A comparison of anti-STEC activity among several Bifidobacterium strains with natural resistance to streptomycin revealed that strains such as Bifidobacterium bifidum ATCC 15696 and Bifidobacterium catenulatum ATCC 27539(T) did not confer an anti-infectious activity, despite achieving high population levels similar to those of effective strains, such as B. breve strain Yakult and Bifidobacterium pseudocatenulatum DSM 20439. The effective strains produced a high concentration of acetic acid (56 mM) and lowered the pH of the intestine (to pH 6.75) compared to the infected control group (acetic acid concentration, 28 mM; pH, 7.15); these effects were thought to be related to the anti-infectious activity of these strains because the combination of a high concentration of acetic acid and a low pH was found to inhibit Stx production during STEC growth in vitro.
Figures
References
-
- Aiba, Y., H. Ishikawa, K. Shimizu, S. Noda, Y. Kitada, M. Sasaki, and Y. Koga. 2002. Role of internalization in the pathogenicity of Shiga toxin-producing Escherichia coli infection in gnotobiotic murine model. Microbiol. Immunol. 46:723-731. - PubMed
-
- Asahara, T., K. Shimizu, K. Nomoto, M. Watanuki, and R. Tanaka. 2001. Antibacterial effect of fermented milk containing Bifidobacterium breve, Bifidobacterium bifidum and Lactobacillus acidophilus against indigenous Escherichia coli infection in mice. Microb. Ecol. Health Dis. 13:16-24.
-
- Asahara, T., K. Nomoto, K. Shimizu, M. Watanuki, and R. Tanaka. 2001. Increased resistance of mice to Salmonella enterica serovar Typhimurium infection by synbiotic administration of Bifidobacteria and transgalactosylated oligosaccharides. J. Appl. Microbiol. 91:985-996. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

