Cytokine and inducible nitric oxide synthase mRNA expression during experimental murine cryptococcal meningoencephalitis
- PMID: 15039359
- PMCID: PMC375146
- DOI: 10.1128/IAI.72.4.2338-2349.2004
Cytokine and inducible nitric oxide synthase mRNA expression during experimental murine cryptococcal meningoencephalitis
Abstract
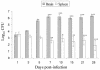
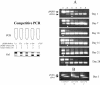
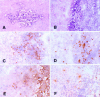
The immune events that take place in the central nervous system (CNS) during cryptococcal infection are incompletely understood. We used competitive reverse transcription-PCR to delineate the time course of the local expression of mRNAs encoding a variety of cytokines and inducible nitric oxide synthase (iNOS) during progressive murine cryptococcal meningoencephalitis and assessed the CNS inflammatory response using immunohistochemistry. Interleukin 18 (IL-18), transforming growth factor beta1, and IL-12p(40) mRNAs were constitutively expressed in the brains of infected and uninfected mice; IL-2 mRNA was not detected at any time. Increased levels of transcripts corresponding to IL-1 alpha, tumor necrosis factor alpha (TNF-alpha), and iNOS were detected as early as day 1 postinfection, with TNF-alpha rising by approximately 30-fold and iNOS increasing by approximately 5-fold by day 7. Each remained at these levels thereafter. IL-4, IL-6, and gamma interferon transcripts were detected on day 5, and IL-1 beta and IL-10 transcripts were detected beginning on day 7. Once detected, each remained at a relatively constant level through 28 days of infection. This cytokine profile does not suggest a polarized Th1 or Th2 response. Immunohistochemistry did not reveal inflammatory infiltrates before day 7, despite the presence of cryptococci. Intraparenchymal abscesses with inflammatory cells in their peripheries were found beginning on day 10. The infiltrates were comprised primarily of cells expressing CD4, CD8, or CD11b; low numbers of cells expressing CD45R/B220 were also present. The persistence of Cryptococcus observed in the CNS may result from an ineffective immune response, perhaps owing to an insufficient anticryptococcal effector function of endogenous glial cells resulting from competing pro- and anti-inflammatory cytokines. These data detail the immune response in the brain and could be important for the future design of specific immunomodulatory therapies for this important opportunistic infection.
Figures


References
-
- Almeida, G. M., R. M. Andrade, and C. A. Bento. 2001. The capsular polysaccharides of Cryptococcus neoformans activate normal CD4(+) T cells in a dominant Th2 pattern. J. Immunol. 167:5845-5851. - PubMed
-
- Ashman, R. B., E. M. Bolitho, and A. Fulurija. 1995. Cytokine mRNA in brain tissue from mice that show strain-dependent differences in the severity of lesions induced by systemic infection with Candida albicans yeast. J. Infect. Dis. 172:823-830. - PubMed
-
- Barluzzi, R., A. Brozzetti, D. Delfino, F. Bistoni, and E. Blasi. 1998. Role of the capsule in microglial cell-Cryptococcus neoformans interaction: impairment of antifungal activity but not of secretory functions. Med. Mycol. 36:189-197. - PubMed
-
- Barluzzi, R., A. Brozzetti, G. Mariucci, M. Tantucci, R. G. Neglia, F. Bistoni, and E. Blasi. 2000. Establishment of protective immunity against cerebral cryptococcosis by means of an avirulent, nonmelanogenic Cryptococcus neoformans strain. J. Neuroimmunol. 109:75-86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

