Fenofibrate for patients with asymptomatic primary biliary cirrhosis
- PMID: 15040040
- PMCID: PMC4727018
- DOI: 10.3748/wjg.v10.i6.894
Fenofibrate for patients with asymptomatic primary biliary cirrhosis
Abstract
Aim: Primary biliary cirrhosis (PBC) is a chronic, cholestatic disease of autoimmune etiology, the histology of which shows a destruction of the intrahepatic bile duct and portal inflammation. Ursodeoxycholic acid (UDCA) is now used as a first-line drug for asymptomatic PBC (aPBC) because it is reported that UDCA decreases mortality and prolongs the time of liver transplantation. However, only 20-30% of patients respond fully to UDCA. Recently, lipoprotein-lowering agents have been found to be effective for PBC. The aim of this study was to examine the safety and efficacy of fenofibrate, a member of the fibrate class of hypolipidemic and anti-inflammatory agent via peroxysome proliferatory-activated receptor alpha, in patients with aPBC.
Methods: Fenofibrate was administered for twelve weeks in nine patients with aPBC who failed to respond to UDCA. UDCA was used along with fenofibrate during the study. The data from aPBC patients were analyzed to assess the biochemical effect of fenofibrate during the study.
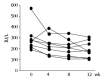
Results: The serum levels of alkaline phosphatase (ALP) (285+/-114.8 IU/L) and immunoglobulin M (IgM) (255.8+/-85.9 mg/dl) significantly decreased to 186.9+/-76.2 IU/L and 192.9+/-67.5 mg/dL respectively, after fenofibrate treatment in patients with aPBC (P<0.05). Moreover, the titer of antimitochondrial antibody (AMA) also decreased in 4 of 9 patients with aPBC. No adverse reactions were observed in any patients.
Conclusion: Fenofibrate appears to be significantly effective in treating patients with aPBC who respond incompletely to UDCA alone. Although the mechanism of fenofibrate on aPBC has not yet been fully clarified, combination therapy using fenofibrate and UDCA might be related to the anti-immunological effects, such as the suppression of AMA production as well as its anti-inflammatory effect.
Figures
References
-
- Poupon RE, Poupon R, Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group. N Engl J Med. 1994;330:1342–1347. - PubMed
-
- Poupon R, Poupon RE. Treatment of primary biliary cirrhosis. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:615–628. - PubMed
-
- Combes B, Carithers RL, Maddrey WC, Lin D, McDonald MF, Wheeler DE, Eigenbrodt EH, Muñoz SJ, Rubin R, Garcia-Tsao G. A randomized, double-blind, placebo-controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1995;22:759–766. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical