Metallothionein can function as a chaperone for zinc uptake transport into prostate and liver mitochondria
- PMID: 15041247
- PMCID: PMC4464834
- DOI: 10.1016/j.jinorgbio.2004.02.005
Metallothionein can function as a chaperone for zinc uptake transport into prostate and liver mitochondria
Abstract
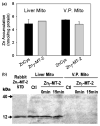
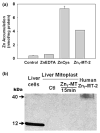
In mammalian cells the cytosolic concentration of free Zn(2+) ions is extremely low (nM-fM range) and unlikely to provide an adequate pool for the uptake and accumulation of zinc in mitochondria. We previously identified a mitochondrial uptake transport process that effectively transports zinc directly from low molecular weight zinc ligands independent of and in the absence of available free Zn(2+) ions. Since metallothionein (MT) is an important ligand form of cellular zinc, we determined if Zn(7)-MT was an effective chaperone and donor for delivery and uptake of zinc by prostate and liver mitochondria. The results reveal that both intact mitochondria and mitoplasts effectively accumulated zinc from Zn(7)-MT. The study confirms and extends our previous report that the putative zinc transporter is associated with the inner mitochondrial membrane and involves a direct exchange of zinc from the ligand to the transporter. The ventral prostate cells contain no detectable MT; so that ligands (such as citrate, aspartate) other than MT are zinc donors for mitochondrial zinc accumulation. However, in liver and perhaps other cells, Zn(7)-MT is probably important in the cytosolic trafficking of zinc to the mitochondria for the uptake of zinc into the mitochondrial matrix by the putative zinc uptake transporter.
Figures
References
-
- Outten CE, O’Halloran TV. Science. 2001;292:2488–2492. - PubMed
-
- Hathout Y, Fabrisa D, Fenselau C. Internat J Mass Spectrom. 2001;204:1–6.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

