Galanin acts as a neuroprotective factor to the hippocampus
- PMID: 15041741
- PMCID: PMC387381
- DOI: 10.1073/pnas.0304823101
Galanin acts as a neuroprotective factor to the hippocampus
Abstract
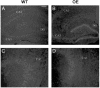
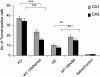
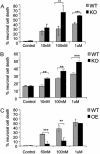
The expression of the neuropeptide galanin is markedly up-regulated in many areas of the central and peripheral nervous system after injury. We have recently demonstrated that peripheral sensory neurons depend on galanin for neurite extension after injury, mediated by activation of the second galanin receptor subtype (GALR2). We therefore hypothesized that galanin might also act in a similar manner in the CNS, reducing cell death in hippocampal models of excitotoxicity. Here we report that galanin acts an endogenous neuroprotective factor to the hippocampus in a number of in vivo and in vitro models of injury. Kainate-induced hippocampal cell death was greater in both the CA1 and CA3 regions of galanin knockout animals than in WT controls. Similarly, exposure to glutamate or staurosporine induced significantly more neuronal cell death in galanin knockout organotypic and dispersed primary hippocampal cultures than in WT controls. Conversely, less cell death was observed in the hippocampus of galanin overexpressing transgenic animals after kainate injection and in organotypic cultures after exposure to staurosporine. Further, exogenous galanin or the previously described high-affinity GALR2 agonist, both reduced cell death when coadministered with glutamate or staurosporine in WT cultures. These results demonstrate that galanin acts an endogenous neuroprotective factor to the hippocampus and imply that a galanin agonist might have therapeutic uses in some forms of brain injury.
Figures


References
-
- Tatemoto, K., Rokaeus, A., Jornvall, H., McDonald, T. J. & Mutt, V. (1983) FEBS Lett. 164, 124-128. - PubMed
-
- Misane, I., Razani, H., Wang, F. H., Jansson, A., Fuxe, K. & Ogren, S. O. (1998) Eur. J. Neurosci. 10, 1230-1240. - PubMed
-
- Pieribone, V. A., Xu, Z. Q., Zhang, X., Grillner, S., Bartfai, T. & Hokfelt, T. (1995) Neuroscience 64, 861-874. - PubMed
-
- Hokfelt, T., Xu, Z. Q., Shi, T. J., Holmberg, K. & Zhang, X. (1998) Ann. N.Y. Acad. Sci. 863, 252-263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

