Virus entry: molecular mechanisms and biomedical applications
- PMID: 15043007
- PMCID: PMC7097642
- DOI: 10.1038/nrmicro817
Virus entry: molecular mechanisms and biomedical applications
Abstract
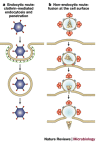
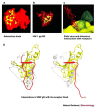
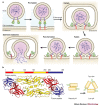
Viruses have evolved to enter cells from all three domains of life--Bacteria, Archaea and Eukaryotes. Of more than 3,600 known viruses, hundreds can infect human cells and most of those are associated with disease. To gain access to the cell interior, animal viruses attach to host-cell receptors. Advances in our understanding of how viral entry proteins interact with their host-cell receptors and undergo conformational changes that lead to entry offer unprecedented opportunities for the development of novel therapeutics and vaccines.
Conflict of interest statement
The author declares no competing financial interests.
Figures


References
-
- d'Herelle F. The Bacteriophage and its Behavior. 1926.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

