Animal models of bone metastasis
- PMID: 15043188
- PMCID: PMC3057671
- DOI: 10.1007/978-1-4419-9129-4_3
Animal models of bone metastasis
Abstract
Animal models will continue to be indispensable to investigate the pathogenesis of bone metastasis in vivo, conduct preclinical chemotherapeutic, chemoprevention and genetic therapy studies, test gene delivery mechanisms, and identify metastasis suppressor and inducer genes. It is likely that the bone marrow microenvironment, such as the endothelial cells, stromal cells, hematopoietic cells, bone cells, and the intercellular matrix play important roles in the localization and clonal growth of cancer cells in bone. Given the complexity of bone metastasis, many genes are expected to be involved in the pathogenesis and few are likely indispensable. The use of genomic and proteomic approaches to study these animal models will identify key targets for therapeutic intervention. As we further refine these models and use imaging for real-time evaluation of cells, and eventually target genes, these models will more closely mirror human disease and will hopefully become more predictive of the human response to therapy.
Figures

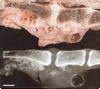


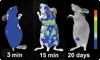
Similar articles
-
Third North American Symposium on Skeletal Complications of Malignancy: summary of the scientific sessions.Cancer. 2003 Feb 1;97(3 Suppl):719-25. doi: 10.1002/cncr.11136. Cancer. 2003. PMID: 12548568
-
Animal models of bone metastasis.Cancer. 2003 Feb 1;97(3 Suppl):748-57. doi: 10.1002/cncr.11150. Cancer. 2003. PMID: 12548572 Review.
-
Evaluation, prognosis, and medical treatment considerations of metastatic bone tumors.Orthopedics. 1992 May;15(5):589-96. doi: 10.3928/0147-7447-19920501-10. Orthopedics. 1992. PMID: 1589351 Review.
-
Medical management of skeletal metastasis.Neurosurg Clin N Am. 2004 Oct;15(4):529-36, xi. doi: 10.1016/j.nec.2004.04.015. Neurosurg Clin N Am. 2004. PMID: 15450887 Review.
-
Treatment of pathological humerus shaft fractures with intramedullary nails with or without cement fixation.Arch Orthop Trauma Surg. 2011 Apr;131(4):503-8. doi: 10.1007/s00402-010-1172-6. Epub 2010 Aug 26. Arch Orthop Trauma Surg. 2011. PMID: 20740287
Cited by
-
Advances in optical imaging and novel model systems for cancer metastasis research.Clin Exp Metastasis. 2007;24(8):699-705. doi: 10.1007/s10585-007-9115-5. Epub 2007 Oct 31. Clin Exp Metastasis. 2007. PMID: 17972147 Review.
-
What is the Best Radionuclide for Immuno-PET of Multiple Myeloma? A Comparison Study Between 89Zr- and 64Cu-Labeled Anti-CD138 in a Preclinical Syngeneic Model.Int J Mol Sci. 2019 May 24;20(10):2564. doi: 10.3390/ijms20102564. Int J Mol Sci. 2019. PMID: 31137758 Free PMC article.
-
Stem cells and bone diseases: new tools, new perspective.Bone. 2015 Jan;70:55-61. doi: 10.1016/j.bone.2014.09.009. Epub 2014 Sep 18. Bone. 2015. PMID: 25240458 Free PMC article. Review.
-
miR-429 inhibits migration and invasion of breast cancer cells in vitro.Int J Oncol. 2015 Feb;46(2):531-8. doi: 10.3892/ijo.2014.2759. Epub 2014 Nov 17. Int J Oncol. 2015. PMID: 25405387 Free PMC article.
-
MTA1 drives malignant progression and bone metastasis in prostate cancer.Mol Oncol. 2018 Sep;12(9):1596-1607. doi: 10.1002/1878-0261.12360. Epub 2018 Aug 14. Mol Oncol. 2018. PMID: 30027683 Free PMC article.
References
-
- Yoneda T, Williams PJ, Myoi A, Michigami T, Mbalaviele G. Cellular and molecular mechanisms of development of skeletal metastases. In: Body J-J, editor. Tumor bone diseases and osteoporosis in cancer patients. New York: Marcel Dekker; 1999. pp. 41–69.
-
- Cher ML. Mechanisms governing bone metastasis in prostate cancer. Curr Opin Urol. 2001;11:483–488. - PubMed
-
- van der Pluijm G, Sijmons B, Vloedgraven H, Deckers M, Papapoulos S, Lowik C. Monitoring metastatic behavior of human tumor cells in mice with species-specific polymerase chain reaction: elevated expression of angiogenesis and bone resorption stimulators by breast cancer in bone metastases. J Bone Miner Res. 2001;16:1077–1091. - PubMed
-
- Sung V, Cattell DA, Bueno JM, et al. Human breast cancer cell metastasis to long bone and soft organs of nude mice: a quantitative assay. Clin Exp Metastasis. 1997;15:173–183. - PubMed
-
- Nemeth JA, Harb JF, Barroso U, Jr, He Z, Grignon DJ, Cher ML. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res. 1999;59:1987–1993. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
