Novel neuronal phenotypes from neural progenitor cells
- PMID: 15044527
- PMCID: PMC3242437
- DOI: 10.1523/JNEUROSCI.4161-03.2004
Novel neuronal phenotypes from neural progenitor cells
Abstract
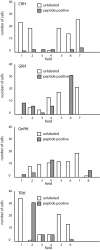
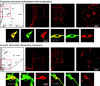
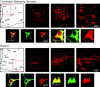
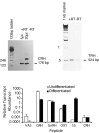
We report the first isolation of progenitor cells from the hypothalamus, a derivative of the embryonic basal plate that does not exhibit neurogenesis postnatally. Neurons derived from hypothalamic progenitor cells were compared with those derived from progenitor cultures of hippocampus, an embryonic alar plate derivative that continues to support neurogenesis in vivo into adulthood. Aside from their different embryonic origins and their different neurogenic potential in vivo, these brain regions were chosen because they are populated with cells of three different categories: Category I cells are generated in both hippocampus and hypothalamus, Category II cells are generated in the hypothalamus but are absent from the hippocampus, and Category III is a cell type generated in the olfactory placode that migrates into the hypothalamus during development. Stem-like cells isolated from other brain regions, with the ability to generate neurons and glia, produce neurons of several phenotypes including gabaergic, dopaminergic, and cholinergic lineages. In the present study, we extended our observations into neuroendocrine phenotypes. The cultured neural precursors from 7-week-old rat hypothalamus readily generated neuropeptide-expressing neurons. Hippocampal and hypothalamic progenitor cultures converged to indistinguishable populations and produced neurons of all three categories, confirming that even short-term culture confers or selects for immature progenitors with enough plasticity to elaborate neuronal phenotypes usually inhibited in vivo by the local microenvironment. The range of phenotypes generated from neuronal precursors in vitro now includes the peptides found in the neuroendocrine system: corticotropin-releasing hormone, growth hormone-releasing hormone, gonadotropin-releasing hormone, oxytocin, somatostatin, thyrotropin-releasing hormone, and vasopressin.
Figures





References
-
- Alvarez-Bolado G, Swanson LW (1996) Developmental brain maps: structure of the embryonic rat brain. Amsterdam: Elsevier.
-
- Alvarez-Buylla A (1990) Mechanism of neurogenesis in adult avian brain. Experientia 46: 948–955. - PubMed
-
- Barry J, Dubois MP, Puolain P (1973) LRF producing cells of the mammalian hypothalamus. Z Zellforsch 146: 351–366. - PubMed
-
- Bennett-Clarke C, Joseph SA (1982) Immunocytochemical distribution of LHRH neurons and processes in the rat: hypothalamic and extrahypothalamic locations. Cell Tissue Res 221: 493–504. - PubMed
-
- Bloch B, Brazeau P, Ling N, Bohlen P, Esch F, Wherenberg WB, Benoit R, Bloom F, Guillemin R (1983) Immunohistochemical detection of growth hormone-releasing factor in brain. Nature 301: 607–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
