Group III metabotropic glutamate receptors maintain tonic inhibition of excitatory synaptic input to hypocretin/orexin neurons
- PMID: 15044540
- PMCID: PMC6729849
- DOI: 10.1523/JNEUROSCI.5416-03.2004
Group III metabotropic glutamate receptors maintain tonic inhibition of excitatory synaptic input to hypocretin/orexin neurons
Abstract
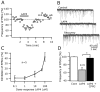
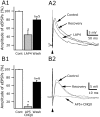
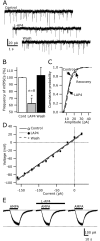
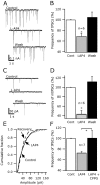
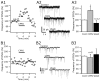
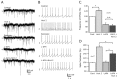
Hypocretin/orexin neurons play an important role in hypothalamic arousal. Synaptic glutamate input to hypocretin neurons regulates cell firing. We studied the actions of group III metabotropic glutamate receptors (mGluRs) in modulating the activity of hypocretin neurons using whole-cell voltage- and current-clamp recording in mouse whole hypothalamic slices or minislices consisting only of the lateral hypothalamus. Selective green fluorescent protein expression was used to detect live hypocretin neurons. The mGluR agonist l-(+)-2-amino-4-phosphonobutyric acid (l-AP-4) inhibited synaptic input to hypocretin neurons in a dose-dependent manner; both spontaneous glutamate and GABA-mediated synaptic currents were reduced in frequency. l-AP-4 also reduced the amplitude of postsynaptic potentials evoked by a stimulating electrode placed medial or lateral to the recorded cell. No postsynaptic effect of l-AP-4 was found relative to membrane potential, input resistance, or AMPA-evoked currents. l-AP-4 appeared to act by a presynaptic mechanism and reduced the frequency of both glutamate- and GABA-mediated miniature events recorded in the presence of tetrodotoxin, with no change in amplitude. (RS)-phosphonopentanoic acid (CPPG), a group III mGluR antagonist, suppressed the actions of l-AP-4. Of substantial interest, CPPG by itself increased synaptic activity recorded in hypocretin neurons, suggesting an ongoing inhibitory tone attributable to activation of group III mGluRs. Glutamatergic interneurons have been suggested to play a role in a positive feedback recruitment of hypocretin on hypocretin neurons. l-AP-4 blocked hypocretin-mediated increases in EPSCs and attenuated the hypocretin-mediated increase in spike frequency. Together, these data suggest that tonically active inhibitory mGluRs are expressed on local hypocretin-sensitive glutamate neurons within the lateral hypothalamus that modulate the output of the hypocretin arousal system.
Figures

References
-
- Abrahamson EE, Moore RY (2001) The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res 889: 1–22. - PubMed
-
- Awad-Granko H, Conn PJ (2001) Activation of groups I or III metabotropic glutamate receptors inhibits excitatory transmission in the rat subthalamic nucleus. Neuropharmacology 41: 32–34. - PubMed
-
- Bergles DE, Jahr CE (1997) Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 19: 1297–1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
