Sensory neuron signaling to the brain: properties of transmitter release from olfactory nerve terminals
- PMID: 15044541
- PMCID: PMC6729835
- DOI: 10.1523/JNEUROSCI.5745-03.2004
Sensory neuron signaling to the brain: properties of transmitter release from olfactory nerve terminals
Abstract
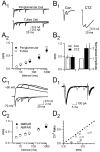
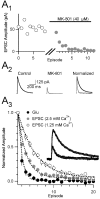
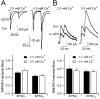
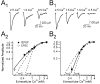
Olfactory receptor neurons (ORNs) convey sensory information directly to the CNS via conventional glutamatergic synaptic contacts in olfactory bulb glomeruli. To better understand the process by which information contained in the odorant-evoked firing of ORNs is transmitted to the brain, we examined the properties of glutamate release from olfactory nerve (ON) terminals in slices of the rat olfactory bulb. We show that marked paired pulse depression is the same in simultaneously recorded periglomerular and tufted neurons, and that this form of short-term plasticity is attributable to a reduction of glutamate release from ON terminals. We used the progressive blockade of NMDA receptor (NMDAR) EPSCs by MK-801 [(5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-10-imine hydrogen maleate] and stationary fluctuation analysis of AMPA receptor (AMPAR) EPSCs to determine the probability of release (P(r)) of ON terminals; both approaches indicated that P(r) is unusually high (>/=0.8). The low-affinity glutamate receptor antagonists gamma-d-glutamylglycine and l-amino-5-phosphonovaleric acid blocked ON-evoked AMPAR- and NMDAR-mediated EPSCs, respectively, to the same extent under conditions of low and high P(r), suggesting that multivesicular release is not a feature of ON terminals. Although release from most synapses exhibits a highly nonlinear dependence on extracellular Ca(2+), we find that the relationship between glutamate release and extracellular Ca(2+) at ON terminals is nearly linear. Our results suggest that ON terminals have specialized features that may contribute to the reliable transmission of sensory information from nose to brain.
Figures

References
-
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT (2000) Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol 84: 1194–1203. - PubMed
-
- Bellingham MC, Walmsley B (1999) A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron 23: 159–170. - PubMed
-
- Borst JG, Sakmann B (1996) Calcium influx and transmitter release in a fast CNS synapse. Nature 383: 431–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
