Dynamics of coilin in Cajal bodies of the Xenopus germinal vesicle
- PMID: 15044688
- PMCID: PMC387330
- DOI: 10.1073/pnas.0401106101
Dynamics of coilin in Cajal bodies of the Xenopus germinal vesicle
Abstract
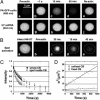
Cajal bodies (CBs) are complex organelles found in the nuclei of a wide variety of organisms, including vertebrates, invertebrates, plants, and yeast. In most cell types CBs are <1 microm in diameter, severely limiting the range of experimental observations that can be made on them. By contrast, CBs in the amphibian oocyte nucleus (also called the germinal vesicle) are 2-10 microm in diameter. We have taken advantage of this large size to carry out kinetic studies on coilin, a protein that is specifically enriched in CBs. We labeled coilin with photoactivatable green fluorescent protein and analyzed the movement of the protein by confocal microscopy in unfixed germinal vesicles isolated in oil. We showed that coilin leaves the CB relatively slowly (minutes rather than seconds) with kinetics similar to earlier measurements on its entrance. We also showed that coilin diffuses very slowly within the CB, consistent with its being in a large macromolecular complex. Finally, we found that the movement of coilin is not directly affected by the transcriptional state of the nucleus or ongoing nucleocytoplasmic exchange. These data on the kinetics of coilin reinforce the conclusion that CB components are in a constant state of flux, consistent with models that postulate an active role for CBs in nuclear physiology.
Figures
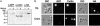

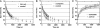
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

