Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets
- PMID: 15044705
- PMCID: PMC397444
- DOI: 10.1073/pnas.0308054101
Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets
Abstract
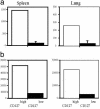
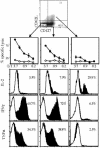
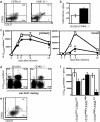
Several recent studies have demonstrated that T-helper cell-dependent events during the initial priming period are required for the generation of CD8(+) T cell-mediated protective immunity. The underlying mechanisms of this phenomenon have not yet been determined, mostly because of difficulties in studying memory T cells or their precursor populations at early stages during immune responses. We identified IL-7 receptor (CD127) surface expression as a marker for long-living memory T cells, most importantly allowing the distinction between memory and effector T cells early after in vivo priming. The combination of surface staining for CD127 and CD62L further separates between two functionally distinct memory cell subsets, which are similar (if not identical) to cell subsets recently described as central memory T cells (CD127(high) and CD62L(high)) and peripheral effector memory T cells (CD127(high) and CD62L(low)). Using this new tool of memory T cell analysis, we demonstrate that CD8(+) T cell priming in the absence of T cell help or CD40L specifically alters the generation of the effector memory T cell subset, which appears to be crucial for immediate memory responses and long-term maintenance of effective protective immunity. Our data reveal a unique strategy to obtain information about the quality of long-term protective immunity early during an immune response, a finding that may be applied in a variety of clinical settings, including the rapid monitoring of vaccination success.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

