Biological functions of the disulfides in bovine pancreatic deoxyribonuclease
- PMID: 15044724
- PMCID: PMC2280041
- DOI: 10.1110/ps.03438204
Biological functions of the disulfides in bovine pancreatic deoxyribonuclease
Abstract
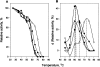
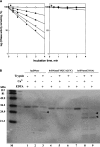
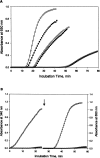
We characterized the biochemical functions of the small nonessential (C101-C104) and the large essential (C173-C209) disulfides in bovine pancreatic (bp) DNase using alanine mutants [brDNase(C101A)] and [brDNase(C173A) and brDNase(C209A)], respectively. We also characterized the effects of an additional third disulfide [brDNase(F192C/A217C)]. Without the Ca(2+) protection, bpDNase and brDNase(C101A) were readily inactivated by trypsin, whereas brDNase(F192C/A217C) remained active. With Ca(2+), all forms of DNase, except for brDNase(C101A), were protected against trypsin. All forms of DNase, after being dissolved in 6 M guanidine-HCl, were fully reactivated by diluting into a Ca(2+)-containing buffer. However, when diluted into a Ca(2+)-free buffer, bpDNase and brDNase(C101A) remained inactive, but 60% of the bpDNase activity was restored with brDNase(F192C/A217C). When heated, bpDNase was inactivated at a transition temperature of 65 degrees C, brDNase(C101A) at 60 degrees C, and brDNase(F192C/A217C) at 73 degrees C, indicating that the small disulfide, albeit not essential for activity, is important for the structural integrity, and that the introduction of a third disulfide can further stabilize the enzyme. When pellets of brDNase(C173A) and brDNase(C209A) in inclusion bodies were dissolved in 6 M guanidine-HCl and then diluted into a Ca(2+)-containing buffer, 10%-18% of the bpDNase activity was restored, suggesting that the "essential" disulfide is not absolutely crucial for enzymatic catalysis. Owing to the structure-based sequence alignment revealing homology between the "nonessential" disulfide of bpDNase and the active-site motif of thioredoxin, we measured 39% of the thioredoxin-like activity for bpDNase based on the rate of insulin precipitation (DeltaA650nm/min). Thus, the disulfides in bpDNase not only play the role of stabilizing the protein molecule but also may engage in biological functions such as the disulfide/dithiol exchange reaction.
Figures



References
-
- Arii, Y., Takahashi, N., Tatsumi, E., and Hirose, M. 1999. Structural properties of recombinant ovalbumin and its transformation into a thermostabilized form by alkaline treatment. Biosci. Biotechnol. Biochem. 63 1392–1399. - PubMed
-
- Baker, A., Payne, C.M., Brieh, M.M., and Powis, G. 1997. Thioredoxin, a gene found overexpressed in human cancer, inhibits apoptosis in vitro and in vivo. Cancer Res. 57 5162–5167. - PubMed
-
- Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72 248–254. - PubMed
-
- Campbell, V.W. and Jackson, D.A. 1980. The effect of divalent cations on the mode of action of DNase I. The initial reaction products produced from covalently closed circular DNA. J. Biol. Chem. 255 3726–3735. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

