Prolonged follow-up of patients in the U.S. multicenter trial of ursodeoxycholic acid for primary biliary cirrhosis
- PMID: 15046215
- PMCID: PMC3891562
- DOI: 10.1111/j.1572-0241.2004.04047.x
Prolonged follow-up of patients in the U.S. multicenter trial of ursodeoxycholic acid for primary biliary cirrhosis
Abstract
Objective: Randomized, double-blind, placebo-controlled trials of ursodeoxycholic acid (UDCA) in patients with primary biliary cirrhosis (PBC) have not demonstrated improvement in survival during the placebo-controlled phases of these trials. Analyses purporting to demonstrate a survival advantage of UDCA are largely dependent on data obtained after the placebo phases were terminated, and placebo-treated patients were offered open-label UDCA. After completion of our 2-yr placebo-controlled trial of UDCA in which we observed no survival benefit for UDCA, we provided the patients with open-label UDCA to see if delay in providing UDCA for 2 yr had any effect on subsequent liver transplantation or death without liver transplantation.
Methods: In our previously reported 2-yr placebo-controlled trial, 151 patients with PBC were randomized to receive either UDCA (n = 77) or placebo (n = 74). The number of patients who progressed to liver transplantation or death without transplantation were similar in both the groups, 12 (16%) in the UDCA-treated and 11 (15%) in placebo-treated patients. All the patients were then offered open-label UDCA, with 61 original UDCA and 56 original placebo-treated patients now taking UDCA in an extended open-label phase of the trial.
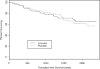
Results: No significant differences were observed in the number of patients who underwent liver transplantation or died without liver transplantation in the open-label phase of the trial. Moreover, no difference in the time to these endpoints was seen over the period of observation of as long as 6 yr from the time of initial randomization.
Conclusions: Results of open-label extensions of previous conducted placebo-controlled trials of UDCA in PBC leave uncertain whether UDCA impacts significantly on liver transplantation and death without liver transplantation in patients with PBC.
Figures

Comment in
-
Ursodeoxycholic acid and long-term survival in primary biliary cirrhosis.Am J Gastroenterol. 2004 Feb;99(2):269-70. Am J Gastroenterol. 2004. PMID: 15046216 No abstract available.
References
-
- Poupon RE, Poupon R, Balkau B, for the UDCA-PBC Study Group Ursodiol for the long-term treatment of primary biliary cirrhosis. New Engl J Med. 1994;330:1342–7. - PubMed
-
- Poupon RE, Balkau B, Eschwege E, et al. The UDCA-PBC Study Group A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. New Engl J Med. 1991;324:1548–51. - PubMed
-
- Lindor KD, Dickson ER, Baldus WP, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284–90. - PubMed
-
- Heathcote EJ, Cauch-Dudek K, Walker V, et al. The Canadian multicenter double-blind randomized controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1994;19:1149–56. - PubMed
-
- Poupon RE, Lindor KD, Cauch-Dudek K, et al. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

