Proton control of oxidation and spin state in a series of iron tripodal imidazole complexes
- PMID: 15046517
- PMCID: PMC1995556
- DOI: 10.1021/ic0351747
Proton control of oxidation and spin state in a series of iron tripodal imidazole complexes
Abstract
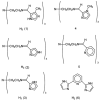
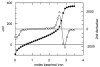
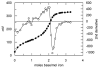
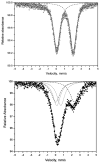
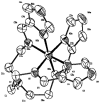
Reaction of iron salts with three tripodal imidazole ligands, H(3)(1), H(3)(2), H(3)(3), formed from the condensation of tris(2-aminoethyl)amine (tren) with 3 equiv of an imidazole carboxaldehyde yielded eight new cationic iron(III) and iron(II), [FeH(3)L](3+or2+), and neutral iron(III), FeL, complexes. All complexes were characterized by EA(CHN), IR, UV, Mössbauer, mass spectral techniques and cyclic voltammetry. Structures of three of the complexes, Fe(2).3H(2)O (C(18)H(27)FeN(10)O(3), a = b = c = 20.2707(5), cubic, I3d, Z = 16), Fe(3).4.5H(2)O (C(18)H(30)FeN(10)O(4.5), a = 20.9986(10), b = 11.7098(5), c = 19.9405(9), beta = 109.141(1), monoclinic, P2(1)/c), Z = 8), and [FeH(3)(3)](ClO(4))(2).H(2)O (C(18)H(26)Cl(2)FeN(10)O(9), a = 9.4848(4), b = 23.2354(9), c = 12.2048(5), beta = 111.147(1) degrees, monoclinic, P2(1)/n, Z = 4) were determined at 100 K. The structures are similar to one another and feature an octahedral iron with facial coordination of imidazoles and imine nitrogen atoms. The iron(III) complexes of the deprotonated ligands, Fe(1), Fe(2), and Fe(3), are low-spin while the protonated iron(III) cationic complexes, [FeH(3)(1)](ClO(4))(3) and [FeH(3)(2)](ClO(4))(3), are high-spin and spin-crossover, respectively. The iron(II) cationic complexes, [FeH(3)(1)]S(4)O(6), [FeH(3)(2)](ClO(4))(2), [FeH(3)(3)](ClO(4))(2), and [FeH(3)(3)][B(C(6)H(5))(4)](2) exhibit spin-crossover behavior. Cyclic voltammetric measurements on the series of complexes show that complete deprotonation of the ligands produces a negative shift in the Fe(III)/Fe(II) reduction potential of 981 mV on average. Deprotonation in air of either cationic iron(II) or iron(III) complexes, [FeH(3)L](3+or2+), yields the neutral iron(III) complex, FeL. The process is reversible for Fe(3), where protonation of Fe(3) yields [FeH(3)(3)](2+).
Figures
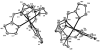
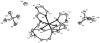
Similar articles
-
Synthesis and characterization of homo- and heterodinuclear M(II)-M'(III) (M(II) = Mn or Fe, M'(III) = Fe or Co) mixed-valence supramolecular pseudo-dimers. The effect of hydrogen bonding on spin state selection of M(II).Dalton Trans. 2011 Jan 7;40(1):181-94. doi: 10.1039/c0dt01098g. Epub 2010 Nov 19. Dalton Trans. 2011. PMID: 21103467
-
Synthesis, X-ray crystal structure, and redox and electronic properties of iron(III)-polyimidazole complexes relevant to the metal sites of iron proteins.Inorg Chem. 2003 Mar 24;42(6):1895-900. doi: 10.1021/ic020401a. Inorg Chem. 2003. PMID: 12639122
-
Accessibility and selective stabilization of the principal spin states of iron by pyridyl versus phenolic ketimines: modulation of the 6A1 ↔ 2T2 ground-state transformation of the [FeN4O2]+ chromophore.Inorg Chem. 2012 Aug 6;51(15):8241-53. doi: 10.1021/ic300732r. Epub 2012 Jul 18. Inorg Chem. 2012. PMID: 22808945
-
57Fe Mössbauer Spectroscopy as a Tool for Study of Spin States and Magnetic Interactions in Inorganic Chemistry.Molecules. 2021 Feb 18;26(4):1062. doi: 10.3390/molecules26041062. Molecules. 2021. PMID: 33670484 Free PMC article. Review.
-
Hexakis(urea-O)iron Complex Salts as a Versatile Material Family: Overview of Their Properties and Applications.ACS Omega. 2024 Mar 4;9(10):11148-11167. doi: 10.1021/acsomega.3c09635. eCollection 2024 Mar 12. ACS Omega. 2024. PMID: 38496982 Free PMC article. Review.
Cited by
-
Use of Pyrazole Hydrogen Bonding in Tripodal Complexes to Form Self Assembled Homochiral Dimers.Materials (Basel). 2020 Mar 31;13(7):1595. doi: 10.3390/ma13071595. Materials (Basel). 2020. PMID: 32244503 Free PMC article.
-
Facile concerted proton-electron transfers in a ruthenium terpyridine-4'-carboxylate complex with a long distance between the redox and basic sites.J Am Chem Soc. 2008 Jun 11;130(23):7210-1. doi: 10.1021/ja801672w. Epub 2008 May 14. J Am Chem Soc. 2008. PMID: 18479096 Free PMC article.
-
Synthesis and characterization of manganese(II) and iron(III) d5 tripodal imidazole complexes. Effect of oxidation state, protonation state and ligand conformation on coordination number and spin state.Dalton Trans. 2006 Feb 28;(8):1009-19. doi: 10.1039/b513282g. Epub 2005 Dec 23. Dalton Trans. 2006. PMID: 16474886 Free PMC article.
-
Bis(2,6-pyrazolyl)pyridines as a New Scaffold for Coordination Polymers.Molecules. 2023 May 23;28(11):4275. doi: 10.3390/molecules28114275. Molecules. 2023. PMID: 37298750 Free PMC article.
-
Dynamic Complex-to-Complex Transformations of Heterobimetallic Systems Influence the Cage Structure or Spin State of Iron(II) Ions.Angew Chem Int Ed Engl. 2020 Feb 17;59(8):3195-3200. doi: 10.1002/anie.201914629. Epub 2020 Jan 9. Angew Chem Int Ed Engl. 2020. PMID: 31788925 Free PMC article.
References
-
- O’Brien P, Sweigart DA. Inorg Chem. 1985;24:1405–1409.
-
- Traylor TG. Acc Chem Res. 1981;14:102–109.
-
- Slattery AJ, Blaho JK, Lehnes J, Goldsby KA. Coord Chem Rev. 1998;174:391–416.
-
- Takeuchi KJ, Samuels GJ, Gersten SW, Meyer TJ. Inorg Chem. 1983:1407–1409.
-
- Dobson JC, Meyer TJ. Inorg Chem. 1988;27:3283–3291.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

