Recombinant modified vaccinia virus Ankara expressing a soluble form of glycoprotein B causes durable immunity and neutralizing antibodies against multiple strains of human cytomegalovirus
- PMID: 15047812
- PMCID: PMC374285
- DOI: 10.1128/jvi.78.8.3965-3976.2004
Recombinant modified vaccinia virus Ankara expressing a soluble form of glycoprotein B causes durable immunity and neutralizing antibodies against multiple strains of human cytomegalovirus
Abstract
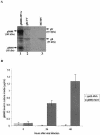
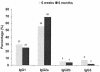
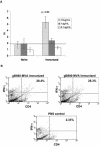
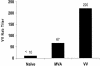
Human cytomegalovirus (CMV) is a viral pathogen that infects both genders, who remain asymptomatic unless they receive immunosuppressive drugs or acquire infections that cause reactivation of latent virus. CMV infection also causes serious birth defects following primary maternal infection during gestation. A safe and effective vaccine to limit disease in this population continues to be elusive. A well-studied antigen is glycoprotein B (gB), which is the principal target of neutralizing antibodies (NAb) towards CMV in humans and has been implicated as the viral partner in the receptor-mediated infection by CMV in a variety of cell types. Antibody-mediated virus neutralization has been proposed as a mechanism by which host immunity could modify primary infection. Towards this goal, an attenuated poxvirus, modified vaccinia virus Ankara (MVA), has been constructed to express soluble CMV gB (gB680-MVA) to induce CMV NAb. Very high levels of gB-specific CMV NAb were produced after two doses of the viral vaccine. NAb were durable within a twofold range for up to 6 months. Neutralization titers developed in immunized mice are equivalent to titers found clinically after natural infection. This viral vaccine, expressing gB derived from CMV strain AD169, induced antibodies that neutralized CMV strains of three different genotypes. Remarkably, preexisting MVA and vaccinia virus (poxvirus) immunity did not interfere with subsequent immunizations of gB680-MVA. The safety characteristics of MVA, combined with the robust immune response to CMV gB, suggest that this approach could be rapidly translated into the clinic.
Figures
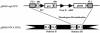


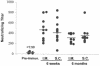
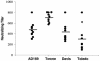

References
-
- Adler, S. P., S. H. Hempfling, S. E. Starr, S. A. Plotkin, and S. Riddell. 1998. Safety and immunogenicity of the Towne strain cytomegalovirus vaccine. Pediatr. Infect. Dis. J. 17:200-206. - PubMed
-
- Adler, S. P., S. A. Plotkin, E. Gonczol, M. Cadoz, C. Meric, J. B. Wang, P. Dellamonica, A. M. Best, J. Zahradnik, S. Pincus, K. Berencsi, W. I. Cox, and Z. Gyulai. 1999. A canarypox vector expressing cytomegalovirus (CMV) glycoprotein B primes for antibody responses to a live attenuated CMV vaccine (Towne). J. Infect. Dis. 180:843-846. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, et al. 2001. Control of a mucosal challenge and preventions of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Andreoni, M., M. Faircloth, L. Vugler, and W. J. Britt. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 23:157-167. - PubMed
-
- Aquino, V. H., and L. T. Figueiredo. 2000. High prevalence of renal transplant recipients infected with more than one cytomegalovirus glycoprotein B genotype. J. Med. Virol. 61:138-142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

