Protection from bacterial infection by a single vaccination with replication-deficient mutant herpes simplex virus type 1
- PMID: 15047818
- PMCID: PMC374270
- DOI: 10.1128/jvi.78.8.4020-4028.2004
Protection from bacterial infection by a single vaccination with replication-deficient mutant herpes simplex virus type 1
Abstract
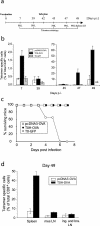
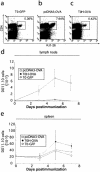
Adaptive immune responses in which CD8(+) T cells recognize pathogen-derived peptides in the context of major histocompatibility complex class I molecules play a major role in the host defense against infection with intracellular pathogens. Cells infected with intracellular bacteria such as Listeria monocytogenes, Salmonella enterica serovar Typhimurium, or Mycobacterium tuberculosis are directly lysed by cytotoxic CD8(+) T cells. For this reason, current vaccines for intracellular pathogens, such as subunit vaccines or viable bacterial vaccines, aim to generate robust cytotoxic T-cell responses. In order to investigate the capacity of a herpes simplex virus type 1 (HSV-1) vector to induce strong cytotoxic effector cell responses and protection from infection with intracellular pathogens, we developed a replication-deficient, recombinant HSV-1 (rHSV-1) vaccine. We demonstrate in side-by-side comparison with DNA vaccination that rHSV-1 vaccination induces very strong CD8(+) effector T-cell responses. While both vaccines provided protection from infection with L. monocytogenes at low, but lethal doses, only rHSV-1 vaccines could protect from higher infectious doses; HSV-1 induced potent memory cytotoxic T lymphocytes that, upon challenge by pathogens, efficiently protected the animals. Despite the stimulation of relatively low humoral and CD4-T-cell responses, rHSV-1 vectors are strong candidates for future vaccine strategies that confer efficient protection from subsequent infection with intracellular bacteria.
Figures




Similar articles
-
Stimulation of protective CD8+ T lymphocytes by vaccination with nonliving bacteria.Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12389-92. doi: 10.1073/pnas.92.26.12389. Proc Natl Acad Sci U S A. 1995. PMID: 8618907 Free PMC article.
-
Human Asymptomatic Epitopes Identified from the Herpes Simplex Virus Tegument Protein VP13/14 (UL47) Preferentially Recall Polyfunctional Effector Memory CD44high CD62Llow CD8+ TEM Cells and Protect Humanized HLA-A*02:01 Transgenic Mice against Ocular Herpesvirus Infection.J Virol. 2017 Jan 3;91(2):e01793-16. doi: 10.1128/JVI.01793-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27847359 Free PMC article.
-
Superior efficacy of secreted over somatic antigen display in recombinant Salmonella vaccine induced protection against listeriosis.Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1458-63. doi: 10.1073/pnas.93.4.1458. Proc Natl Acad Sci U S A. 1996. PMID: 8643654 Free PMC article.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
CD8+ T cells in intracellular bacterial infections of mice.Res Immunol. 1996 Oct-Dec;147(8-9):519-24. doi: 10.1016/s0923-2494(97)85217-0. Res Immunol. 1996. PMID: 9127883 Review.
Cited by
-
MHC class I cross-presentation by dendritic cells counteracts viral immune evasion.Front Immunol. 2012 Nov 26;3:348. doi: 10.3389/fimmu.2012.00348. eCollection 2012. Front Immunol. 2012. PMID: 23189079 Free PMC article.
-
HSV Recombinant Vectors for Gene Therapy.Open Virol J. 2010 Jun 18;4:123-56. doi: 10.2174/1874357901004030123. Open Virol J. 2010. PMID: 20835362 Free PMC article.
-
Nonspecific Effects of Oral Polio Vaccine on Diarrheal Burden and Etiology Among Bangladeshi Infants.Clin Infect Dis. 2017 Aug 1;65(3):414-419. doi: 10.1093/cid/cix354. Clin Infect Dis. 2017. PMID: 28444240 Free PMC article. Clinical Trial.
-
Exploring the Potential of Cytomegalovirus-Based Vectors: A Review.Viruses. 2023 Oct 2;15(10):2043. doi: 10.3390/v15102043. Viruses. 2023. PMID: 37896820 Free PMC article. Review.
-
Susceptibility of human placenta derived mesenchymal stromal/stem cells to human herpesviruses infection.PLoS One. 2013 Aug 5;8(8):e71412. doi: 10.1371/journal.pone.0071412. Print 2013. PLoS One. 2013. PMID: 23940750 Free PMC article.
References
-
- Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. - PubMed
-
- Boursnell, M. E., C. Entwisle, D. Blakeley, C. Roberts, I. A. Duncan, S. E. Chisholm, G. M. Martin, R. Jennings, D. Ni Challanain, I. Sobek, S. C. Inglis, and C. S. McLean. 1997. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J. Infect. Dis. 175:16-25. - PMC - PubMed
-
- Busch, D. H., I. M. Pilip, S. Vijh, and E. G. Pamer. 1998. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8:353-362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

