Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites
- PMID: 15047834
- PMCID: PMC374303
- DOI: 10.1128/jvi.78.8.4185-4196.2004
Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites
Abstract
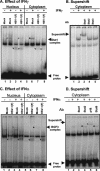
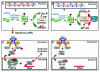
Earlier studies have shown that herpes simplex virus 1 (HSV-1) blocks the interferon response pathways, at least at two sites, by circumventing the effects of activation of protein kinase R by double-stranded RNA and interferon and through the degradation of promyelocytic leukemia protein (PML) since interferon has no antiviral effects in PML(-/-) cells. Here we report on two effects of viral genes on other sites of the interferon signaling pathway. (i) In infected cells, Jak1 kinase associated with interferon receptors and Stat2 associated with the interferon signaling pathway rapidly disappear from infected cells. The level of interferon alpha receptor is also reduced, albeit less drastically at times after 4 h postinfection. Other members of the Stat family of proteins were either decreased in amount or posttranslationally processed in a manner different from those of mock-infected cells. The decrease in the levels of Jak1 and Stat2 may account for the decrease in the formation of complexes consisting of Stat1 or ISGF3 and DNA sequences containing the interferon-stimulated response elements after exposure to interferon. (ii) The disappearance of Jak1 and Stat2 was related at least in part to the function of the virion host shutoff protein, the product of the viral U(L)41 gene. Consistent with this observation, a mutant lacking the U(L)41 gene and treated with interferon produced lesser amounts of a late protein (U(L)38) than the wild-type parent. We conclude that HSV-1 blocks the interferon signaling pathways at several sites.
Figures






References
-
- Aurelian, L., and B. Roizman. 1965. Abortive infection of canine cells by herpes simplex virus. II. The alternative suppression of synthesis of interferon and viral constituents. J. Mol. Biol. 11:539-548. - PubMed
-
- Caldenhoven, E., T. B. van Dijk, R. Solari, J. Armstrong, J. A. Raaijmakers, J. W. Lammers, L. Koenderman, and R. P. de Groot. 1996. STAT3beta, a splice variant of transcription factor STAT3, is a dominant negative regulator of transcription. J. Biol. Chem. 271:13221-13227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

