Rubella virus capsid protein modulates viral genome replication and virus infectivity
- PMID: 15047844
- PMCID: PMC374250
- DOI: 10.1128/jvi.78.8.4314-4322.2004
Rubella virus capsid protein modulates viral genome replication and virus infectivity
Abstract
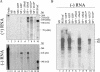
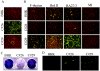
The structural proteins (SP) of the Togaviridae can be deleted in defective interfering RNAs. The dispensability of viral SP has allowed construction of noninfectious viral expression vectors and replicons from viruses of the Alphavirus and Rubivirus genera. Nevertheless, in this study, we found that the SP of rubella virus (RUB) could enhance expression of reporter genes from RUB replicons in trans. SP enhancement required capsid protein (CP) expression and was not due to RNA-RNA recombination. Accumulation of minus- and plus-strand RNAs from replicons was observed in the presence of SP, suggesting that SP specifically affects RNA synthesis. By using replicons containing an antibiotic resistance gene, we found 2- to 50-fold increases in the number of cells surviving selection in the presence of SP. The increases depended significantly on the amount of transfected RNA. Small amounts of RNA or templates that replicated inefficiently showed more enhancement. The infectivity of infectious RNA was increased by at least 10-fold in cells expressing CP. Moreover, virus infectivity was greatly enhanced in such cells. In other cells that expressed higher levels of CP, RNA replication of replicons was inhibited. Thus, depending on conditions, CP can markedly enhance or inhibit RUB RNA replication.
Figures



References
-
- Bol, J. F. 1999. Alfalfa mosaic virus and ilarviruses: involvement of coat protein in multiple steps of the replication cycle. J. Gen. Virol. 80:1089-1102. - PubMed
-
- Derdeyn, C. A. 1994. The characterization of rubella virus defective-interfering RNAs generated during serial undiluted passage and persistent infection. Ph.D. thesis. Georgia State University, Atlanta.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

