Mammalian N-acetylglutamate synthase
- PMID: 15050968
- PMCID: PMC3031861
- DOI: 10.1016/j.ymgme.2003.10.017
Mammalian N-acetylglutamate synthase
Abstract
N-Acetylglutamate synthase (NAGS, E.C. 2.3.1.1) is a mitochondrial enzyme that catalyzes the formation of N-acetylglutamate (NAG), an essential allosteric activator of carbamylphosphate synthetase I (CPSI). The mouse and human NAGS genes have been identified based on similarity to regions of NAGS from Neurospora crassa and cloned from liver cDNA libraries. These genes were shown to complement an argA- (NAGS) deficient Escherichia coli strain, and enzymatic activity of the proteins was confirmed by a new stable isotope dilution assay. The deduced amino acid sequence of mammalian NAGS contains a putative mitochondrial-targeting signal at the N-terminus. The mouse NAGS preprotein was overexpressed in insect cells to determine post-translational modifications and two processed proteins with different N-terminal truncations have been identified. Sequence analysis using a hidden Markov model suggests that the vertebrate NAGS protein contains domains with a carbamate kinase fold and an acyl-CoA N-acyltransferase fold, and protein crystallization experiments are currently underway. Inherited NAGS deficiency results in hyperammonemia, presumably due to the loss of CPSI activity. We, and others, have recently identified mutations in families with neonatal and late-onset NAGS deficiency and the identification of the gene has now made carrier testing and prenatal diagnosis feasible. A structural analog of NAG, carbamylglutamate, has been shown to bind and activate CPSI, and several patients have been reported to respond favorably to this drug (Carbaglu).
Figures


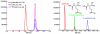
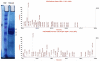
References
-
- Grisolia S, Cohen PP. Catalytic role of glutamate derivatives in citrulline biosynthesis. J. Biol. Chem. 1953;204:753–757. - PubMed
-
- Marshall M, Metzenberg RL, Cohen PP. Purification of carbamyl phosphate synthetase from frog liver. J. Biol. Chem. 1958;233:102–105. - PubMed
-
- Shigesada K, Tatibana M. Enzymatic synthesis of acetylglutamate by mammalian liver preparations and its stimulation by arginine. Biochem. Biophys. Res. Commun. 1971;44:1117–1124. - PubMed
-
- Meijer AJ, Lof C, Ramos IC, Verhoeven AJ. Control of ureagenesis. Eur. J. Biochem. 1985;148:189–196. - PubMed
-
- Tujioka K, Lyou KS, Hirano E, Sano A, Hayase K, Yoshida A, Yokogoshi H. Role of N-acetylglutamate concentration and ornithine transport into mitochondria in urea synthesis of rats given proteins of different quality. J. Agric. Food Chem. 2002;50:7467–7471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

