Modulation of visceral pain and inflammation by protease-activated receptors
- PMID: 15051630
- PMCID: PMC1574902
- DOI: 10.1038/sj.bjp.0705750
Modulation of visceral pain and inflammation by protease-activated receptors
Abstract
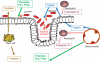
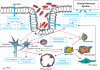
The gastrointestinal (GI) tract is exposed to a large array of proteases, under both physiological and pathophysiological conditions. The discovery of G protein-coupled receptors activated by proteases, the protease-activated receptors (PARs), has highlighted new signaling functions for proteases in the GI tract, particularly in the domains of inflammation and pain mechanisms. Activation of PARs by selective peptidic agonists in the intestine or the pancreas leads to inflammatory events and changes in visceral nociception, suggesting that PARs could be involved in the modulation of visceral pain and inflammation. PARs are present in most of the cells that are potentially actors in the generation of irritable bowel syndrome (IBS) symptoms. Activation of PARs interferes with several pathophysiological factors that are involved in the generation of IBS symptoms, such as altered motility patterns, inflammatory mediator release, altered epithelial functions (immune, permeability and secretory) and altered visceral nociceptive functions. Although definitive studies using genetically modified animals, and, when available, pharmacological tools, in different IBS and inflammatory models have not yet confirmed a role for PARs in those pathologies, PARs appear as promising targets for therapeutic intervention in visceral pain and inflammation processes.
Figures

References
-
- ASOKANANTHAN N., GRAHAM P.T., STEWART D.J., BAKKER A.J., EIDNE K.A., THOMPSON P.J., STEWART G.A. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J. Immunol. 2002;169:4572–4578. - PubMed
-
- BARAU E., DUPONT C. Modifications of intestinal permeability during food provocation procedures in pediatric irritable bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 1990;11:72–77. - PubMed
-
- BURESI M.C., HOLLENBERG M.D., MACNAUGHTON W.K. Activation of protease-activated receptor-1 (PAR-1) inhibits neurally evoked chloride secretion in the mouse colon. Can. J. Gastroenterol. 2003;17:61A. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

