Gating dynamics of the acetylcholine receptor extracellular domain
- PMID: 15051806
- PMCID: PMC2217457
- DOI: 10.1085/jgp.200309004
Gating dynamics of the acetylcholine receptor extracellular domain
Abstract
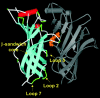
We used single-channel recording and model-based kinetic analyses to quantify the effects of mutations in the extracellular domain (ECD) of the alpha-subunit of mouse muscle-type acetylcholine receptors (AChRs). The crystal structure of an acetylcholine binding protein (AChBP) suggests that the ECD is comprised of a beta-sandwich core that is surrounded by loops. Here we focus on loops 2 and 7, which lie at the interface of the AChR extracellular and transmembrane domains. Side chain substitutions in these loops primarily affect channel gating by either decreasing or increasing the gating equilibrium constant. Many of the mutations to the beta-core prevent the expression of functional AChRs, but of the mutants that did express almost all had wild-type behavior. Rate-equilibrium free energy relationship analyses reveal the presence of two contiguous, distinct synchronously-gating domains in the alpha-subunit ECD that move sequentially during the AChR gating reaction. The transmitter-binding site/loop 5 domain moves first (Phi = 0.93) and is followed by the loop 2/loop 7 domain (Phi = 0.80). These movements precede that of the extracellular linker (Phi = 0.69). We hypothesize that AChR gating occurs as the stepwise movements of such domains that link the low-to-high affinity conformational change in the TBS with the low-to-high conductance conformational change in the pore.
Figures
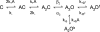










References
-
- Brejc, K., W.J. van Dijk, R.V. Klaassen, M. Schuurmans, J. van Der Oost, A.B. Smit, and T.K. Sixma. 2001. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 411:269–276. - PubMed

